Cefminox sodium compound crystal, preparation method of cefminox sodium compound crystal and sterile powder injection containing cefminox sodium compound crystal
A technology of cefminox sodium and sterile powder injection, which is applied in the fields of medical preparations containing active ingredients, organic chemistry, powder delivery, etc., and can solve problems such as threats to patient safety and anaphylactic shock reactions of cefminox sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
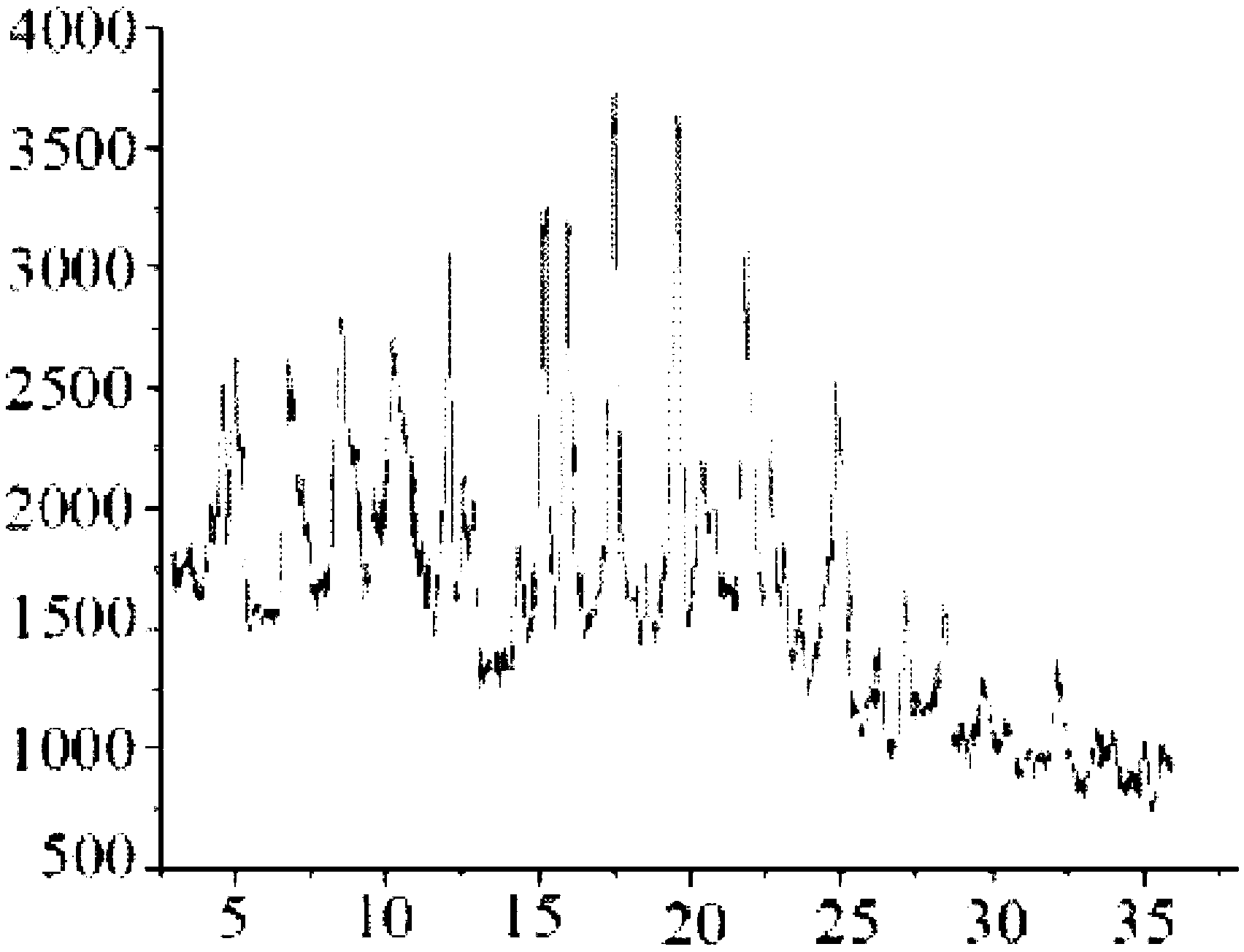
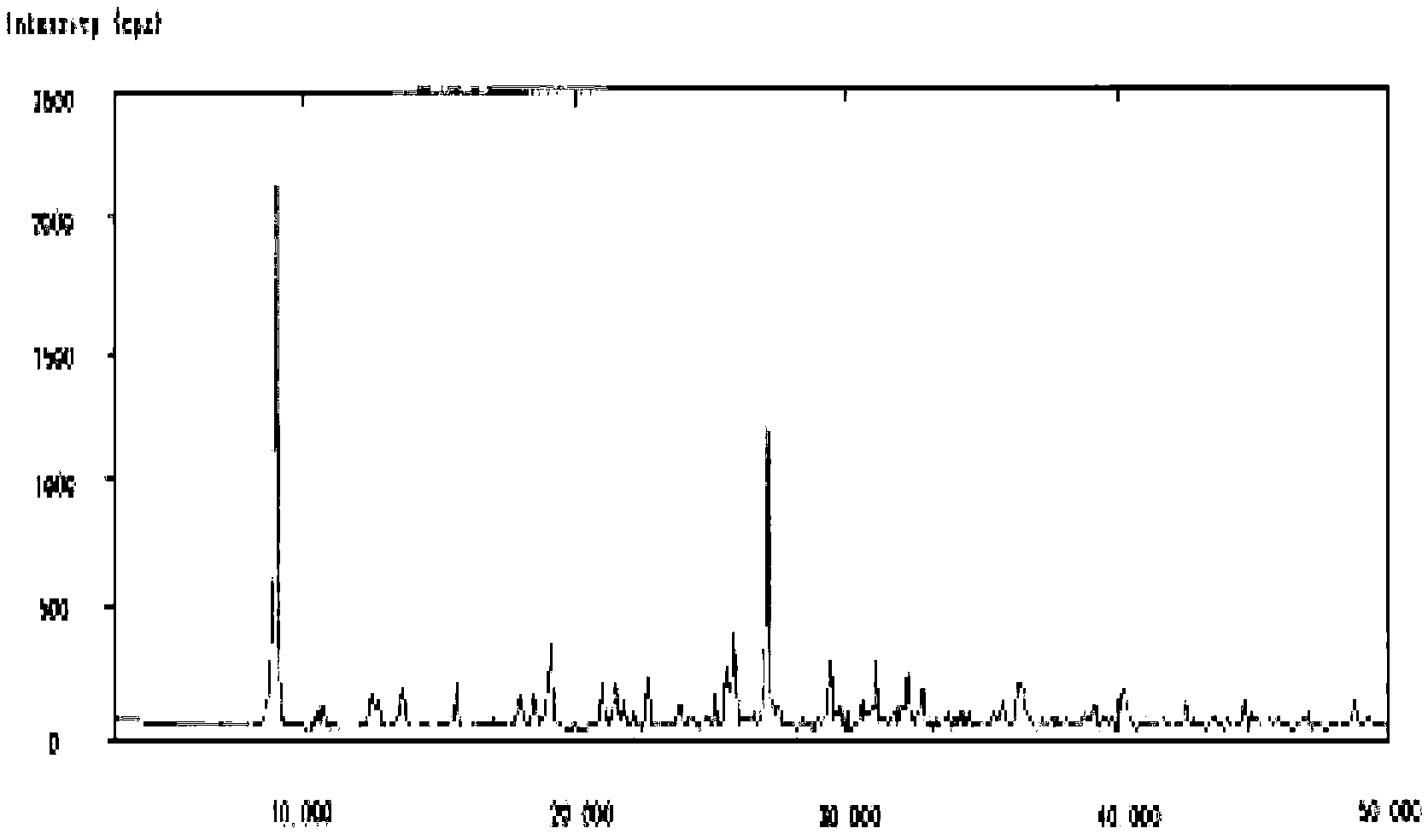
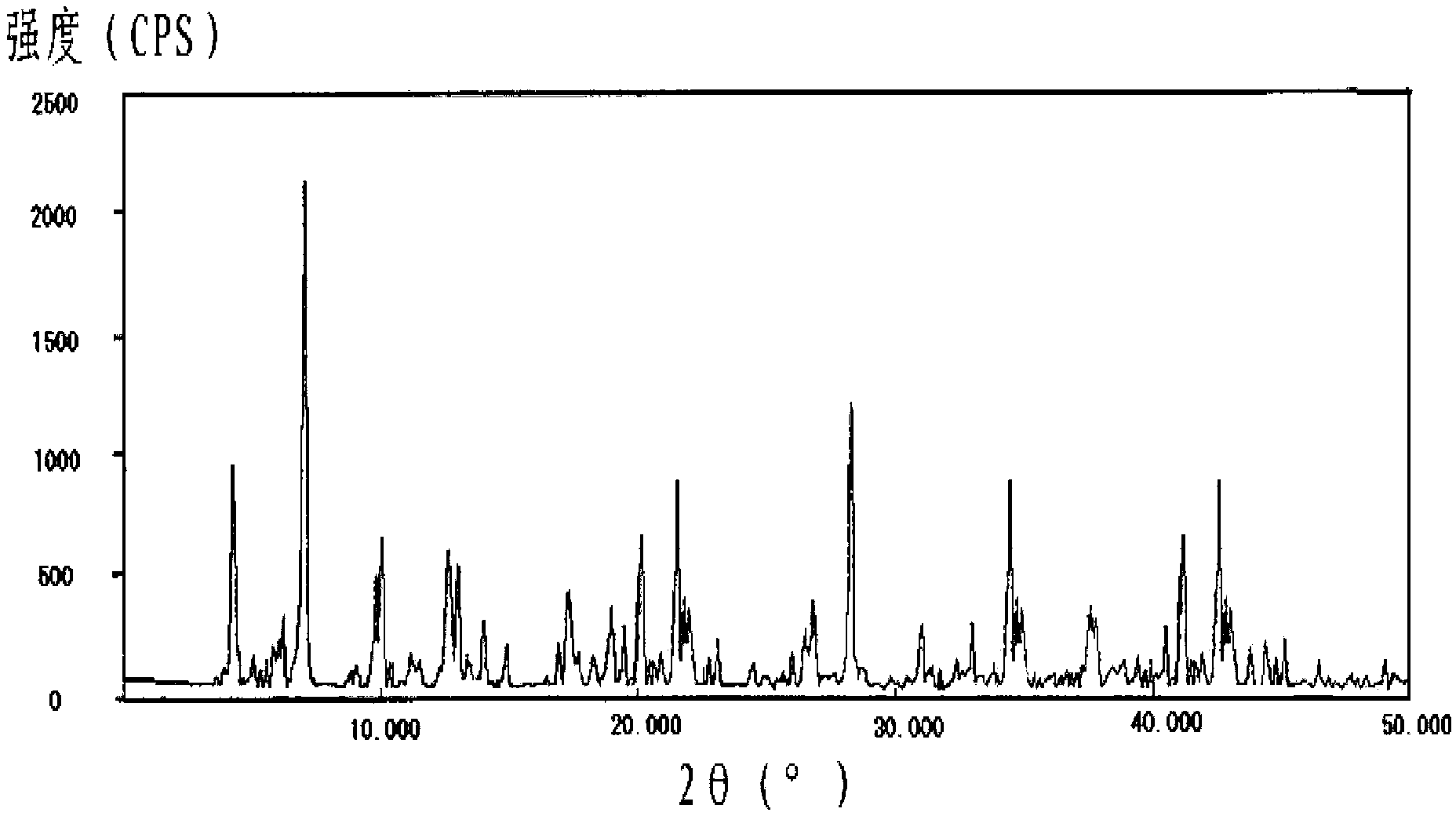
Image
Examples
Embodiment 1
[0061] [Example 1] Prepare cefminox sodium heptahydrate with reference to prior art
[0062] The cefminox sodium raw material is prepared according to the method of Example 4 of the US4357331 manual, and 92g of 7β-bromoacetamido-7α-methoxyl-3-(1-methyl-1H-tetrazol-5-yl)sulfur) Methyl)-3-cephalosporin-4-carboxylic acid was suspended in 1L of water, and saturated aqueous sodium bicarbonate solution was added to adjust its pH to 7.2 at low temperature, and 46.3g of D-cysteine salt was added to the mixed solution salt, cooled, and stirred at room temperature for 30 to 40 minutes, during which time the pH was adjusted to between 7.1 and 7.2. The reaction mixture was subjected to column separation using a 50 x 700 cm column (containing 8000 ml of Diaion HP-20), washed with water, and 52.6 g of cefminox sodium were obtained from the water-eluted fraction.
[0063] Cefminox sodium heptahydrate is prepared according to the method of US4555404 specification example 1, 10.3 g of cefmi...
Embodiment 2
[0066] [embodiment 2] the preparation of cefminox sodium compound crystal
[0067] Under room temperature conditions, drop into 5g cefminox sodium heptahydrate crude product to reactor, add the water dissolving of 50ml, stir 28 minutes, filter, regulate filtrate pH with glacial acetic acid and be 6.3, the temperature of control filtrate is 18 ℃, in every At a stirring speed of 100 rpm, slowly add 200ml of a mixed solution of absolute ethanol and acetone (the volume ratio of absolute ethanol and acetone is 1:3) dropwise at a speed of 420ml / h until the crystals are precipitated, and then at 30 Under constant stirring, control the supersaturated concentration of the solution, continue to slowly add 200ml of n-butanol and tetrahydrofuran mixed solution (the volume ratio of n-butanol and tetrahydrofuran is 2:1), make the crystallization complete, filter, and use 50ml of absolute ethanol and acetone The mixed solution was washed twice, sucked dry, and dried under reduced pressure to...
Embodiment 3
[0070] [embodiment 3] the preparation of cefminox sodium compound crystal
[0071] Under room temperature conditions, drop into 5g cefminox sodium heptahydrate crude product to reactor, add the water dissolving of 50ml, stir 28 minutes, filter, regulate filtrate pH with glacial acetic acid and be 6.9, the temperature of control filtrate is 30 ℃, in every At a stirring speed of 150 revolutions per minute, slowly add 200ml of a mixed solution of absolute ethanol and acetone (the volume ratio of absolute ethanol and acetone is 1:8) dropwise at a speed of 480ml / h until the crystals are precipitated, and then at 70 per minute Under constant stirring, control the supersaturated concentration of the solution, continue to slowly add 600ml of n-butanol and tetrahydrofuran mixed solution (the volume ratio of n-butanol and tetrahydrofuran is 5:1), make the crystallization complete, filter, and use 50ml of absolute ethanol and acetone The mixed solution was washed twice, sucked dry, and d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com