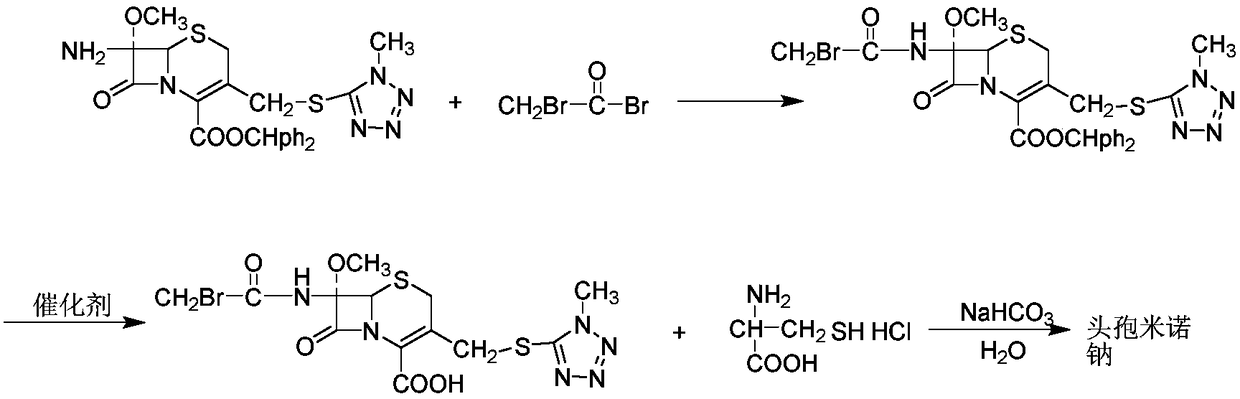
Synthesis method of cefminox sodium
A cefminox sodium and synthetic method technology, applied in the field of drug synthesis, can solve problems such as corrosive reactors, unfavorable industrial production, environmental pollution, etc., and achieve the goals of avoiding acid waste liquid, increasing yield, improving yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 A kind of solid preparation method of phosphoric acid loaded activated carbon
[0033] S1: the aqueous solution of sodium hydroxide of 0.1mol / L and the aqueous solution of hydrochloric acid of 0.1mol / L are successively processed to activated carbon, remove the soluble acid-base impurity of activated carbon surface load, obtain the activated carbon after treatment;
[0034] S2: soak the treated activated carbon in 25% sulfuric acid aqueous solution for 3 hours, filter, and vacuum dry to obtain activated activated carbon;
[0035] S3: Mix the activated carbon with 20% phosphoric acid aqueous solution, react at 40°C for 2 hours, cool to room temperature, impregnate at room temperature for 24 hours, filter, and dry to obtain the phosphoric acid-loaded activated carbon catalyst.
[0036] The mass ratio of activated carbon to 20% phosphoric acid aqueous solution in step S3 is 1:3.5.
Embodiment 2
[0037] Embodiment 2 A kind of preparation method of phosphoric acid loaded activated carbon
[0038] S1: the aqueous solution of sodium hydroxide of 0.1mol / L and the aqueous solution of hydrochloric acid of 0.1mol / L are successively processed to activated carbon, remove the soluble acid-base impurity of activated carbon surface load, obtain the activated carbon after treatment;
[0039] S2: soak the treated activated carbon in 25% sulfuric acid aqueous solution for 3 hours, filter, and vacuum dry to obtain activated activated carbon;
[0040] S3: Mix the activated carbon with 20% phosphoric acid aqueous solution, react at 60° C. for 2 hours, cool to room temperature, impregnate at room temperature for 24 hours, filter, and dry to obtain the phosphoric acid-loaded activated carbon catalyst.
[0041] The mass ratio of activated carbon to 20% phosphoric acid aqueous solution in step S3 is 1:4.
Embodiment 3
[0042] Embodiment 3 A kind of preparation method of phosphoric acid loaded activated carbon
[0043] S1: the aqueous solution of sodium hydroxide of 0.1mol / L and the aqueous solution of hydrochloric acid of 0.1mol / L are successively processed to activated carbon, remove the soluble acid-base impurity of activated carbon surface load, obtain the activated carbon after treatment;
[0044] S2: soak the treated activated carbon in 25% sulfuric acid aqueous solution for 3 hours, filter, and vacuum dry to obtain activated activated carbon;
[0045] S3: Mix the activated carbon with 20% phosphoric acid aqueous solution, react at 50°C for 2 hours, cool to room temperature, impregnate at room temperature for 24 hours, filter, and dry to obtain the phosphoric acid-loaded activated carbon catalyst.
[0046] The mass ratio of activated carbon to 20% phosphoric acid aqueous solution in the step S3 is 1:3.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com