Preparation method for cephamycin intermediate
An intermediate, cephamycin technology, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of complex steps and large recycling pollution, and achieve the effects of reducing costs, less pollution, and ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
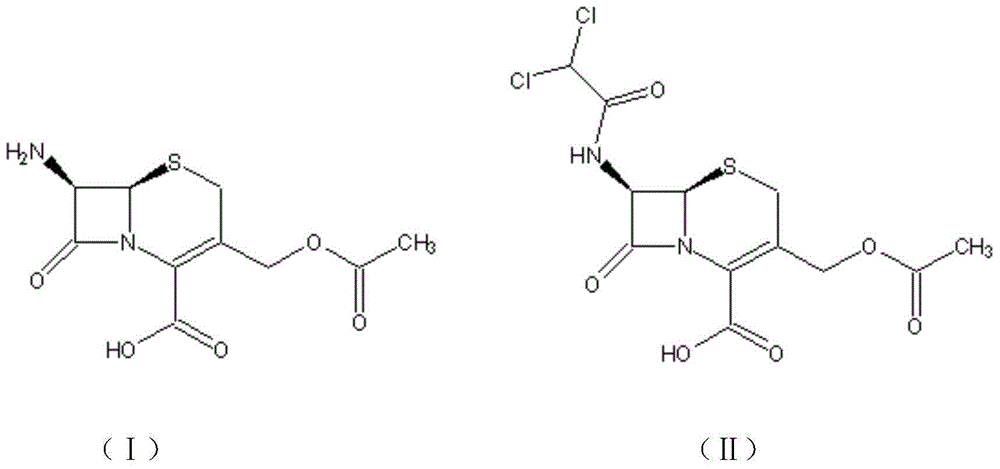
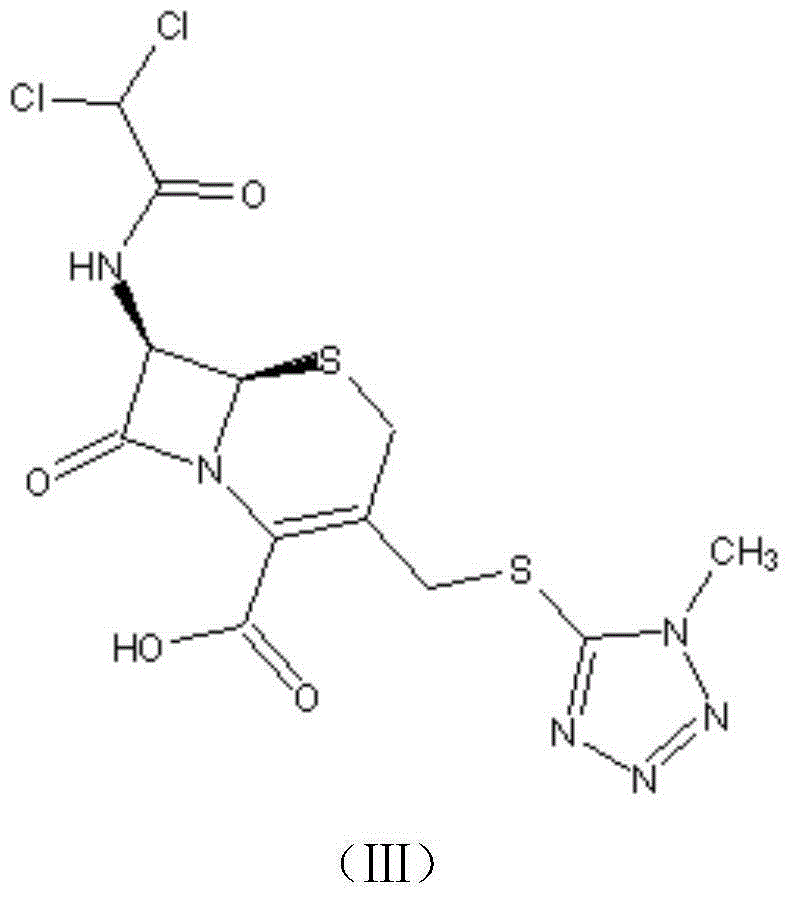
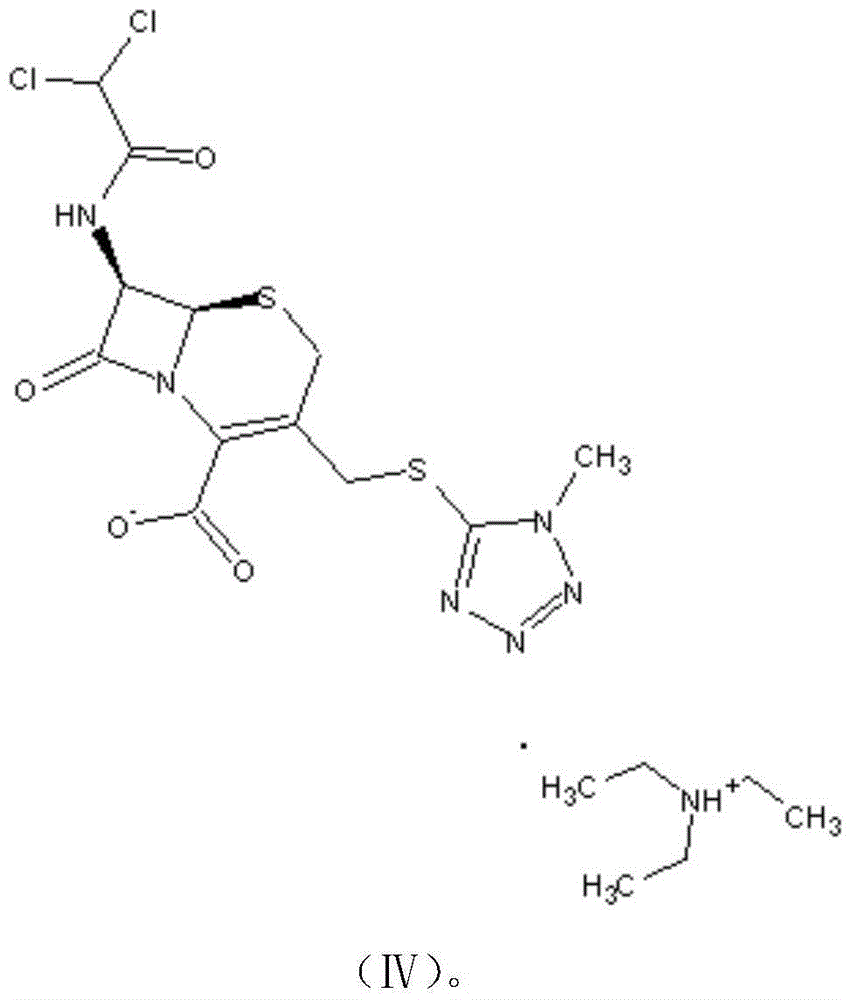
[0041] A preparation method of cephamycin intermediate shown in formula (IV), comprising the steps of:
[0042] (1) Add 10g of 7-ACA raw material into 100ml of water, then add 9.25g of sodium bicarbonate, stir at 30-35°C for 1-2h to dissolve, cool down to 0-5°C, and add 8.14g of dichloroethylene dropwise under temperature control Acyl chloride, keep stirring and reacting at 0-5°C for 30 minutes, adjust the pH to 4.5-5.0 with 30wt% sodium hydroxide, crystallize in large quantities, grow crystals at 0-5°C for 2 hours, filter, wash the filter cake with 15ml water, and the product is 25-5 Dry at 30°C to obtain 13.2 g of the compound of formula (II), the product purity is greater than 98%, and the molar yield: 93.6%.
[0043] (2) Add 3.0g MMT to 100ml ethyl acetate, stir at 10-25°C for 30min to dissolve, add 8.8g of the compound of formula (II) prepared in step (1), then add 0.5g of solid sulfonic acid catalyst PEP-14, heat Heat the reaction at 50°C for 3.5 hours, recover the soli...
Embodiment 2
[0046] Preparation method as described in Example 1, the difference is that,
[0047] Step (1) Add 10g of 7-ACA to 50ml of 30wt% formic acid solution, stir at 20-25°C for 60min to dissolve, cool the feed liquid to 0-5°C, add 8.14g of dichloroacetyl chloride dropwise, and control the temperature at 0-5 Stir and react at ℃ for 30 minutes, adjust the pH to about 4.5-5.0 with 30wt% sodium hydroxide, crystallize in large quantities, grow crystals at 0-5℃ for 2 hours, filter, wash the filter cake with 15ml water, and dry the product in vacuum to obtain compound 8.8 of formula (II) g, purity 93.5%, molar yield: 70.91%.
Embodiment 3
[0049] Preparation method as described in Example 1, the difference is that,
[0050] Ethyl acetate in the step (2) is changed to acetone;
[0051] Finally, 10.5 g of DCT in the form of triethylamine (compound represented by formula (IV)) was obtained, with a purity of 98.2% and a molar yield of 84.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com