D-cysteine hydrochloride monohydrate preparation method
A technology of cysteine hydrochloride and monohydrate, which is applied in the field of preparation of D-cysteine hydrochloride monohydrate and can solve the problem of ring opening of D-4-tetrahydrothiazole-4-carboxylic acid Difficulties, poor optical purity, low product yield, etc., to achieve the effects of high product yield, high chemical purity, and short drying time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
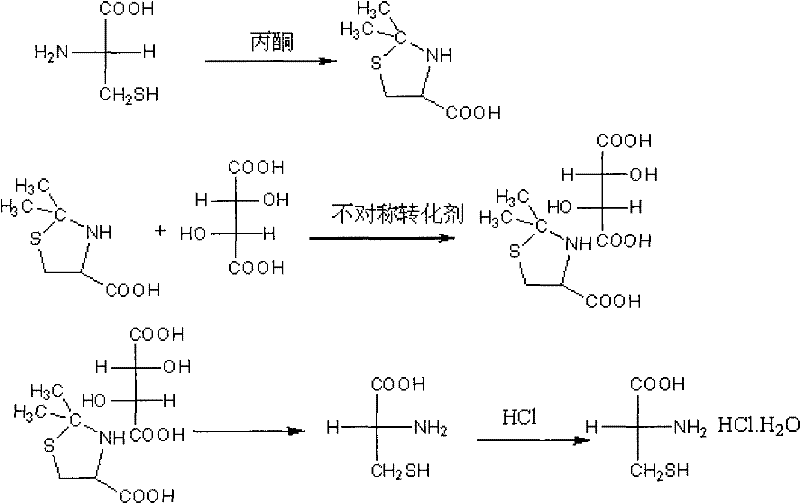
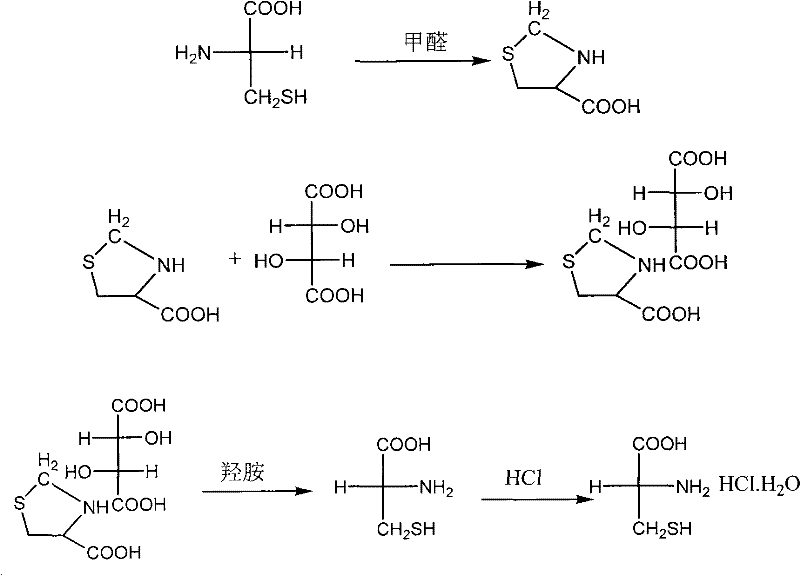
[0036] (1), D-2, the preparation of 2-dimethyltetrahydrothiazole-4-carboxylic acid-L-tartrate double salt:
[0037] Add 384g of L-cysteine, 480g of L-tartaric acid, 1600ml of acetone and 1200ml of acetic acid into the reaction bottle, stir and dissolve and heat to reflux, reflux for 0.5h, then add 26ml of salicylaldehyde, reflux for 20h, cool to 0 with an ice-water bath ℃, filtered, the filter cake was washed twice with the filtrate, and then washed with acetone until it was colorless, and dried to obtain 728g of D-2,2-dimethyltetrahydrothiazole-4-carboxylic acid-L-tartrate double salt.
[0038] (2), the preparation of D-cysteine:
[0039] Dissolve 728g of D-2,2-dimethyltetrahydrothiazole-4-carboxylic acid-L-tartaric acid double salt in 4050ml of water, heat to reflux for 1.5h, then concentrate the reaction solution to about 400ml, adjust the system with 275ml of triethylamine PH = 3.6, then add 2100ml of ethanol, stir for 10 minutes, cool down to 20°C, filter and dry to obta...
Embodiment 2
[0043] (1) Prepare 728g of D-2,2-dimethyltetrahydrothiazole-4-carboxylic acid-L-tartrate double salt according to (1) in Example 1, dissolve in 4050ml of water, heat to reflux for 1.5h, add 35g Activated carbon, stirred for 15 minutes, filtered to remove the activated carbon, concentrated the reaction solution to about 400ml, adjusted the pH of the system to 3.6 with 275ml of triethylamine, then added 2100ml of ethanol, stirred for 10 minutes, cooled to 20°C, filtered, dried to obtain D-cysteine Acid 405.2g.
[0044] (2), the preparation of D-cysteine hydrochloride monohydrate:
[0045] Add 405.2g of the prepared D-cysteine into the mixed solution composed of 400ml concentrated hydrochloric acid, stir and react at 40°C for 30 minutes, then let it stand and cool down to about -5°C, filter the precipitated white crystals, and put them in ice acetone Wash and dry to obtain about 406.2 g of D-cysteine hydrochloride monohydrate. The chemical purity is 99.23%, and the yield ...
Embodiment 3
[0047] (1), D-2, the preparation of 2-dimethyltetrahydrothiazole-4-carboxylic acid-L-tartrate double salt:
[0048] Add 384g of L-cysteine, 480g of L-tartaric acid, 1,600ml of acetone and 1,800ml of propionic acid into the reaction bottle, stir and dissolve and heat to reflux, reflux for 0.5h, then add 26ml of salicylaldehyde, reflux for 20h, and cool down to 0°C, filtered, the filter cake was washed twice with the filtrate, and then washed with acetone until it was colorless, and dried to obtain D-DMTL-TA776g.
[0049] (2), the preparation of D-cysteine hydrochloride monohydrate:
[0050] According to (2) (3) step in example 1, 776gD-2,2-dimethyltetrahydrothiazole-4-carboxylic acid-L-tartrate double salt is prepared to obtain D-cysteine hydrochloride monohydrate 434.6g, chemical purity 99.19%, yield 78.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com