Anti-infective pharmaceutical composition containing cefminox
A technology of cefminox and cefminox sodium, applied in the field of medicine, can solve problems such as unsatisfactory effects, aggravated kidney damage, and accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation of the composition aseptic powder injection of embodiment the present invention
[0018] Prescription 1
[0019] Cefminox Sodium 500g
[0020] Na 2 HPO 4 0.4g
[0021] NaH 2 PO 4 0.1g
[0022] Arginine 50g
[0023]
[0024] A total of 1000 sticks were prepared
[0025] Prescription 2
[0026] Cefminox Sodium 1000
[0027] Na 2 HPO 4 0.3g
[0028] NaH 2 PO 4 9.7g
[0029] Arginine 10g
[0030]
[0031] A total of 1000 sticks were prepared
[0032] Prescription 3
[0033] Cefminox Sodium 2000g
[0034] Na 2 HPO 4 80g
[0035] NaH 2 PO 4 120g
[0036] Arginine 200g
[0037]
[0038] A total of 1000 sticks were prepared
[0039] Prescription 4
[0040] Cefminox Sodium 500g
[0041] Na 2 HPO 4 1g
[004...
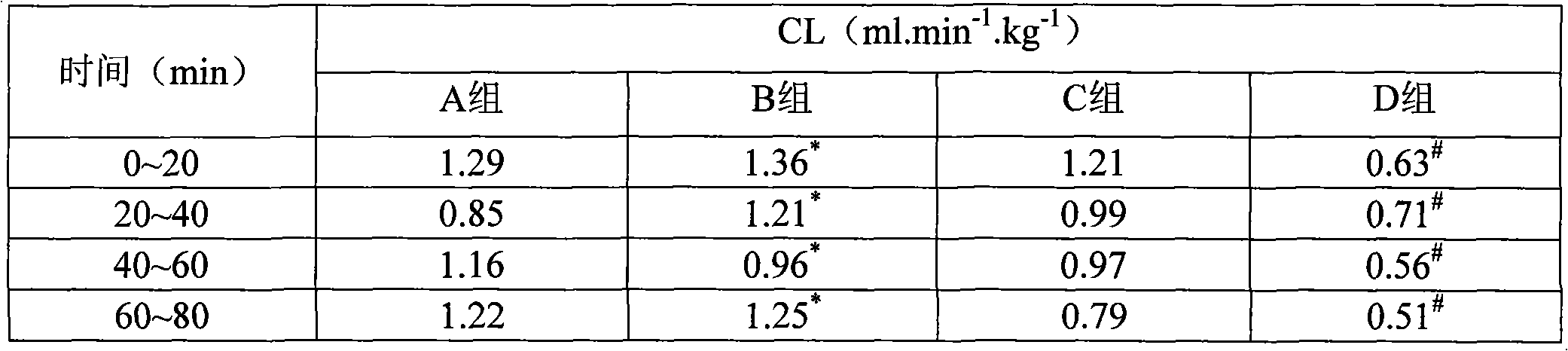
experiment example 1
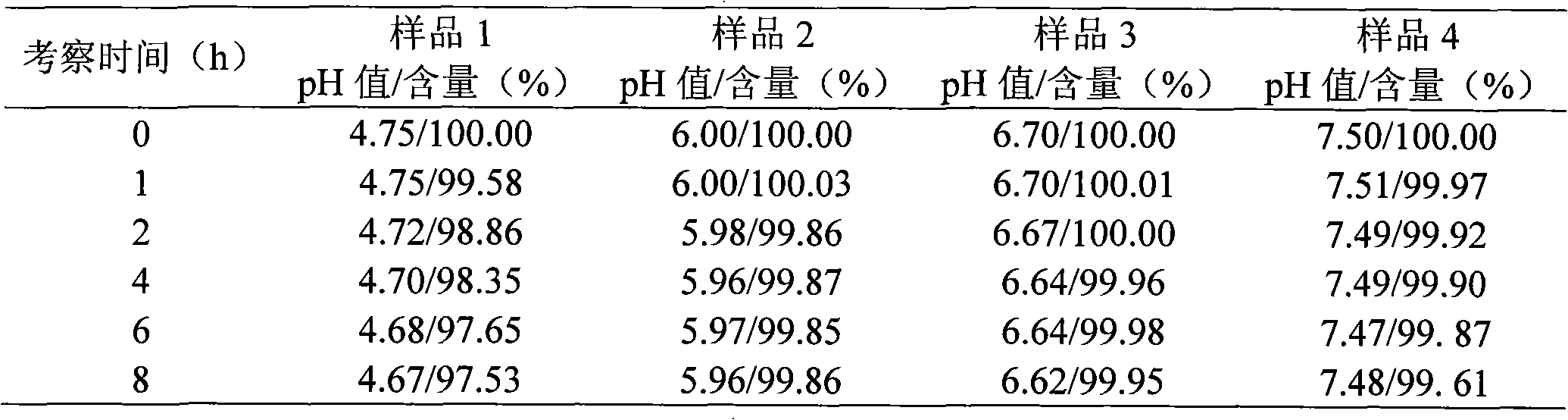
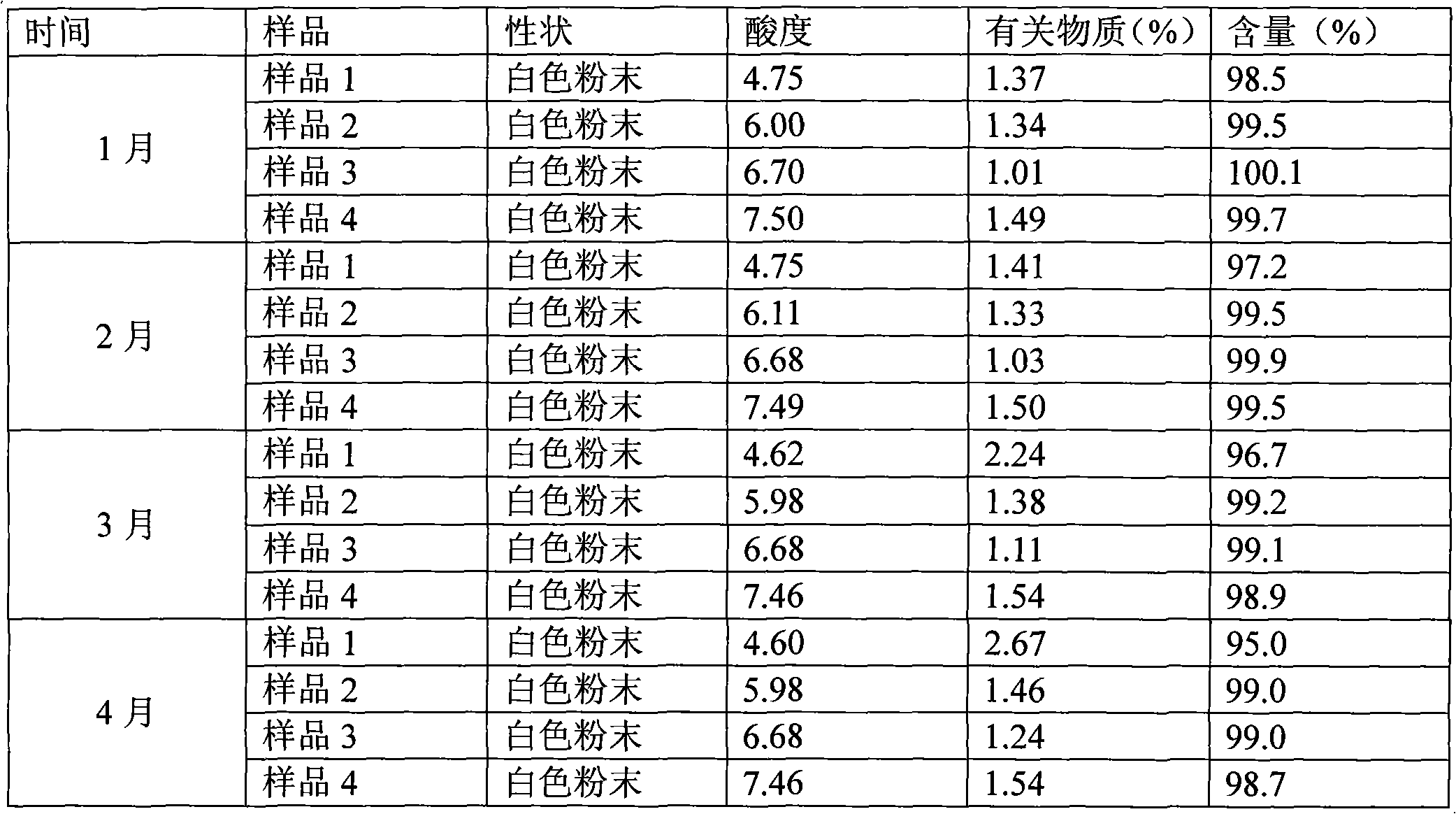
[0065] The investigation of experimental example 1 cefminox sodium solution stability
[0066] Test sample 1, listed cefminox sodium sterile powder injection;
Embodiment 1
[0067] Sample 2, the composition of the present invention, is taken from Example 1 prescription 2;
[0068] Sample 3, the composition of the present invention, is taken from Example 1 prescription 4;
[0069] Sample 4, the composition of the present invention, is taken from prescription 5 of Example 1.
[0070] (1) The influence of pH value on the stability of cefminox sodium
[0071] The experimental method simulates the concentration of routine clinical drugs, prepares a compatible solution of cefminox sodium and sodium chloride infusion with a concentration of 5 mg / mL, and puts it in a biochemical incubator (25±2°C) at 0, 1, 2, 4, 6. Observe the appearance of the solution for 8 hours and measure the pH value. Simultaneously draw the above solution, dilute it into a dilute solution with a concentration of 25 μg / mL, take water as a blank, measure the absorbance at 272nm wavelength, calculate the content of cefminox sodium, and use The content of 0h is 100%, and the relative...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com