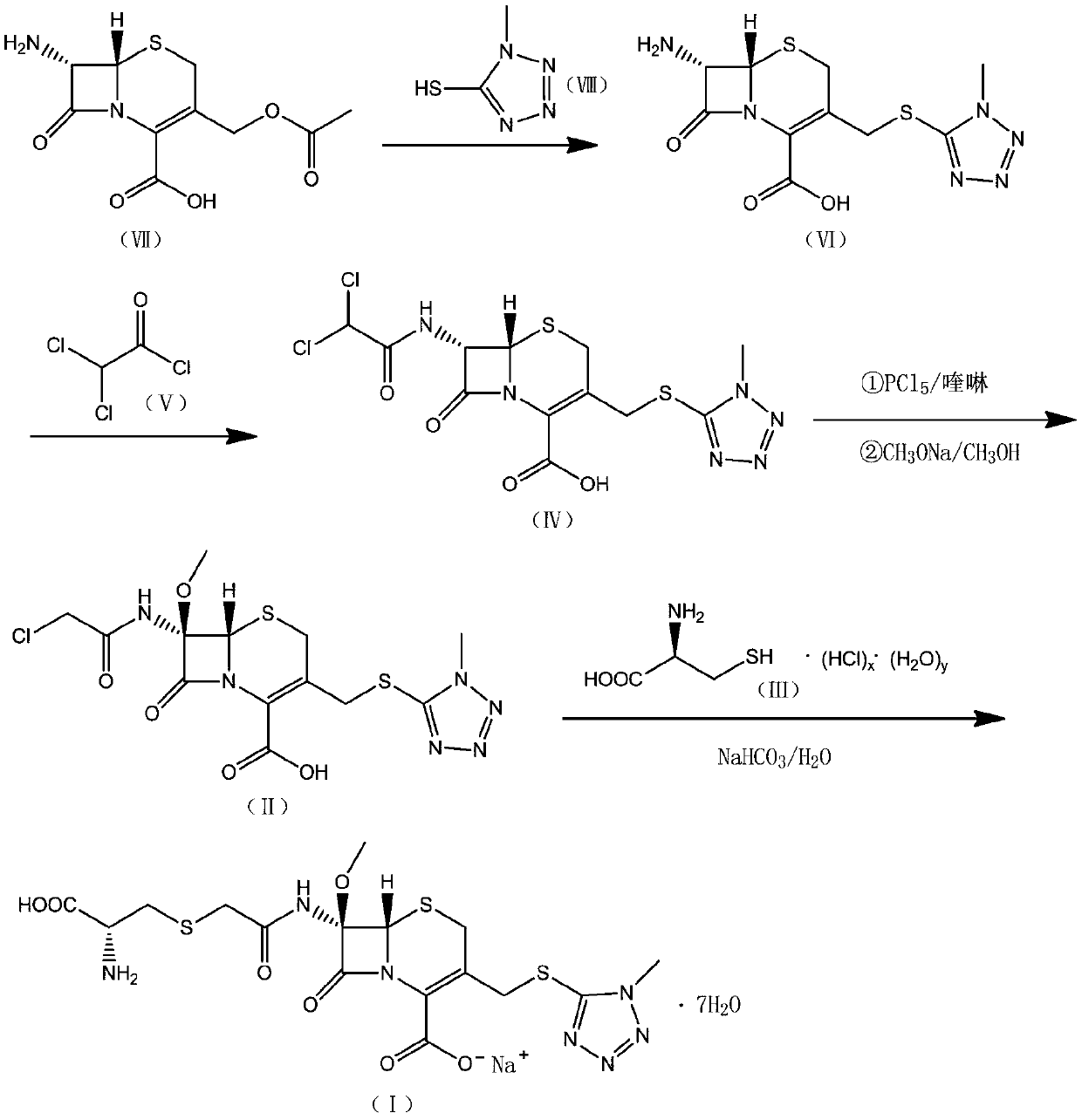
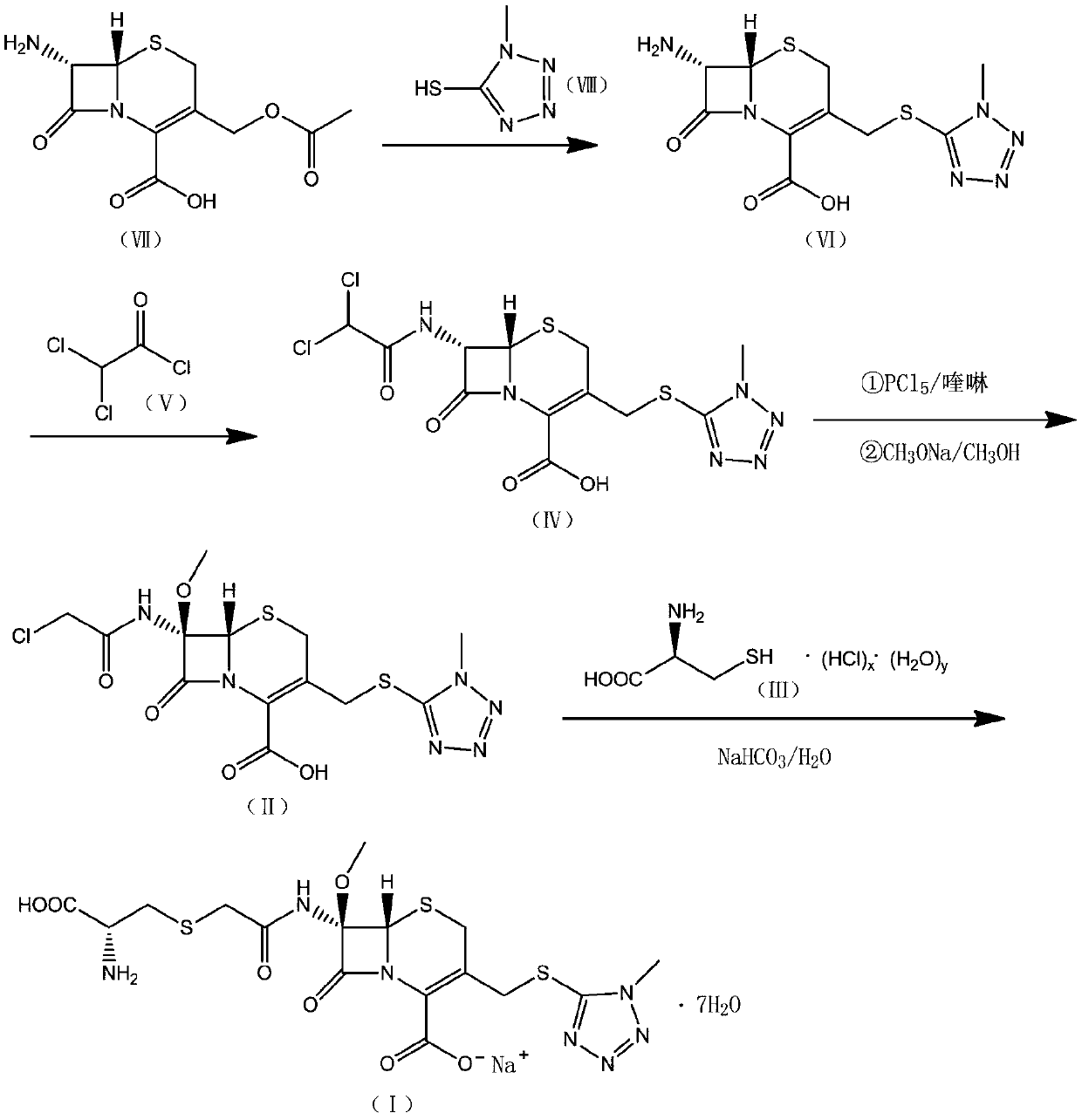
Cefminox sodium preparation method
A technology for cefminox sodium and compound, which is applied in the field of preparation of cefminox sodium and can solve the problems of expensive raw materials, high risk factor, harsh conditions for deprotecting groups and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
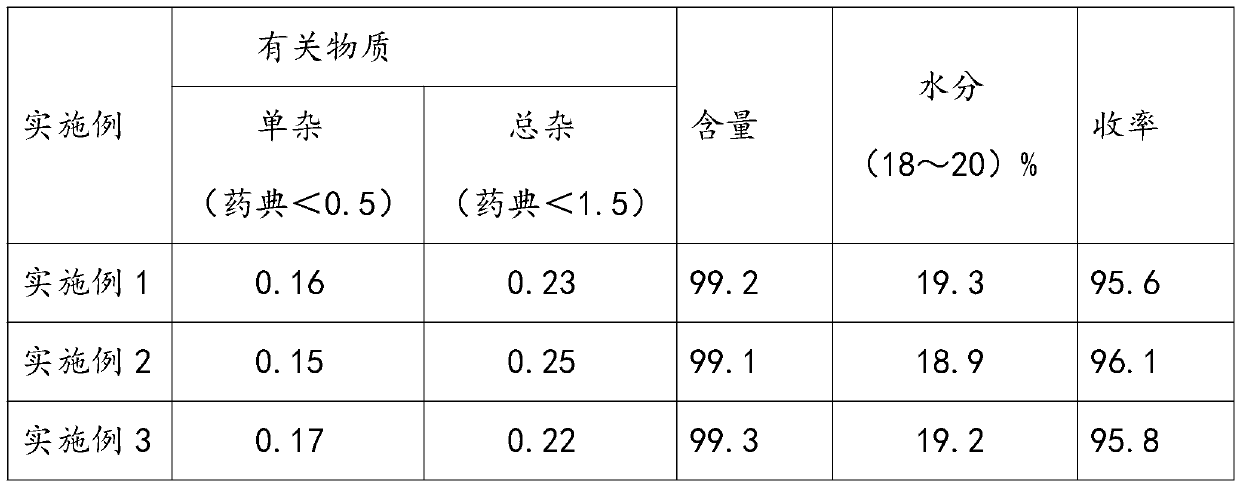
Embodiment 1
[0074] A kind of preparation method of cefminox sodium of the present embodiment, comprises the steps:
[0075] (1) At room temperature, 45g of 7-amino-3-[(acetoxy)methyl]-8-oxo-5-thia-1-azabicyclo[4,2,0]-2-ene -2-carboxylic acid (7-ACA) and 200g dimethyl carbonate were mixed and evenly mixed, 31g 1-methyl 5-mercapto-tetrazolium was added, the temperature was raised to 30°C with stirring, and 128.5g boron trifluoride (40 %), temperature control reaction for 120min, and the reaction is over, slowly drip ammonia water (15%) to the system until the system is turbid, grow the crystal for 30min, suction filter, 210g acetone beating and washing, filter off the solvent, and dry in vacuo to obtain intermediate 7- Amino-3-(1-methyl-1-H-tetraaza-5-thiomethyl)-3-cephem-4-carboxylic acid 46.5 g, yield 118.5%.
[0076] (2) At room temperature, add 36.5g of intermediate 7-amino-3-(1-methyl-1-H-tetraza-5-thiomethyl)-3-cephem-4-carboxylic acid to 195.5g of acetic acid In ethyl ester, stir a...
Embodiment 2
[0080] A kind of preparation method of cefminox sodium of the present embodiment, comprises the steps:
[0081] (1) At room temperature, 40g of 7-amino-3-[(acetoxy)methyl]-8-oxo-5-thia-1-azabicyclo[4,2,0]-2-ene -2-carboxylic acid (7-ACA) and 220g dimethyl carbonate were mixed and evenly mixed, 31.5g 1-methyl 5-mercapto-tetrazolium was added, the temperature was raised to 30°C with stirring, and 127.7g boron trifluoride ( 40%), temperature controlled reaction for 120min, after the reaction was completed, ammonia water (15%) was slowly added dropwise to the system until the system became turbid, the crystal was grown for 30min, suction filtered, 200g of acetone was beaten and washed, the solvent was filtered off, and vacuum-dried to obtain intermediate 7 -Amino-3-(1-methyl-1-H-tetraza-5-thiomethyl)-3-cephem-4-carboxylic acid 48g, yield 120%.
[0082] (2) At room temperature, add 37.5g of intermediate 7-amino-3-(1-methyl-1-H-tetraza-5-thiomethyl)-3-cephem-4-carboxylic acid to 19...
Embodiment 3
[0086] A kind of preparation method of cefminox sodium of the present embodiment, comprises the steps:
[0087] (1) At room temperature, 50g of 7-amino-3-[(acetoxy)methyl]-8-oxo-5-thia-1-azabicyclo[4,2,0]-2-ene -2-carboxylic acid (7-ACA) and 250g dimethyl carbonate were mixed and evenly mixed, 32g 1-methyl 5-mercapto-tetrazolium was added, the temperature was raised to 30°C with stirring, and 125g boron trifluoride (40% ), temperature controlled reaction for 120min, and the reaction was completed, slowly drip ammonia water (15%) to the system until the system was turbid, grow crystals for 30min, filter with suction, wash with 200g of acetone beating, filter to remove the solvent, and dry in vacuo to obtain the intermediate 7-amino - 47 g of 3-(1-methyl-1-H-tetraza-5-thiomethyl)-3-cephem-4-carboxylic acid, yield 119.2%.
[0088] (2) At room temperature, add 38g of intermediate 7-amino-3-(1-methyl-1-H-tetraza-5-thiomethyl)-3-cephem-4-carboxylic acid to 185g of ethyl acetate In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com