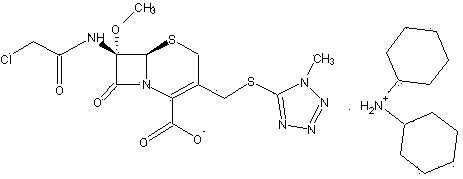
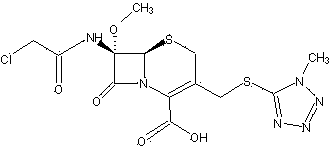
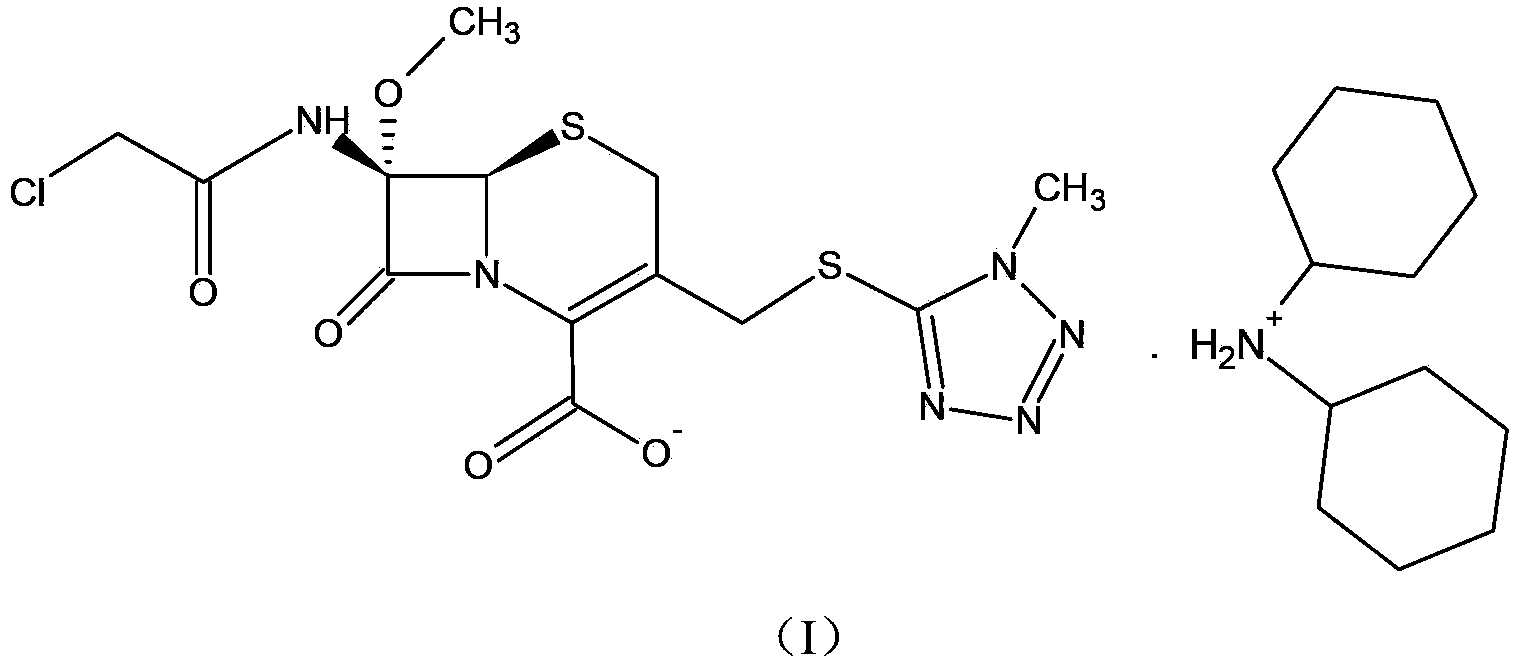
Cephamycin intermediate compound and preparation method thereof
The technology of a compound, cephamycin, is applied in the field of preparation of cephalosporin compounds, which can solve problems such as unfavorable long-term storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, preparation of cephamycin intermediate formula (I) compound
[0032]Dissolve 0.1 mol of the prepared oil containing the compound of formula (II) in 330 ml of acetone, mix thoroughly and cool down to 5°C, slowly add 21.8 g (0.12 mol) of dicyclohexylamine dropwise under stirring, the feed solution It gradually becomes muddy. Keep warm at about 5°C to grow crystals for 2 hours, filter, wash the filter cake with 120ml of acetone, and drain. The obtained solid was vacuum-dried at 40°C until the water content was less than 1%, and 54.2 g of white crystalline powder was obtained, which was the compound of formula (I) of the cephamycin intermediate of the present invention. The yield was 87.9%, and the HPLC purity was 99.53%.
[0033] The structure of the obtained product was confirmed, and the results are as follows:
[0034] IR(KBr):.
[0035] Sample absorption peak / cm -1
vibration type
group
3153
ν N-H
-NH
2939,2858...
Embodiment 2
[0042] Add ethyl acetate to the prepared oil containing 0.1 mol of the compound of formula (II) to prepare an ethyl acetate solution with a concentration of about 100 g / L, cool down to 10°C, and dropwise add 21.8 g of dicyclohexylamine (0.12 mol), add 0.1g of seed crystals, grow the crystals for 3h, cool down to 0°C, continue to grow the crystals for 4h, filter, wash the filter cake with 200ml of ethyl acetate, and drain. The obtained solid was vacuum-dried at 40°C until the water content was less than 1%, and 57.4 g of off-white crystalline powder was obtained, which was the compound of formula (I) of the cephamycin intermediate of the present invention; the yield was 93.1%, and the HPLC purity was 99.27%.
Embodiment 3
[0044] Dissolve 0.1 mol of the oily substance containing the compound of formula (II) in 100 ml of N,N-dimethylformamide, cool down to 5°C, slowly add 21.8 g (0.12 mol) of dicyclohexylamine dropwise, Cultivate the crystal for 2 hours, filter, wash the filter cake with 100ml N,N-dimethylformamide, and drain it. The obtained solid was vacuum-dried at 40°C until the water content was less than 1%, and 53.9 g of white crystalline powder was obtained, which was the compound of formula (I) of the cephamycin intermediate of the present invention. The yield was 87.5%, and the purity of the product was 99.60% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com