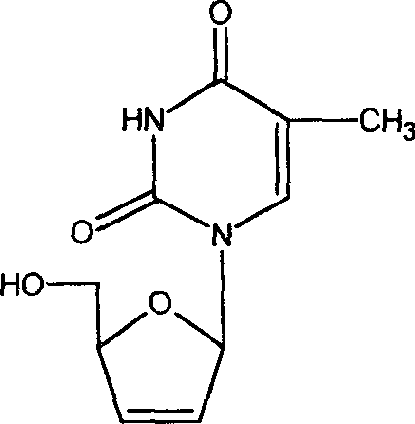
Stavudine sustained release tablet and its preparing process
A technology for stavudine and sustained-release tablets, which is applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc. The probability of release” and the effect of improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Tablet core prescription Raw material Feeding amount Weight ratio
[0028] Stavudine 50g 1
[0029]
[0030] Makes 1000 pieces
[0031] 2. Tablet core preparation process
[0032] (1) Crush stavudine and pass through a 100-mesh sieve. Lactose, microcrystalline cellulose, magnesium stearate, and hypromellose are directly passed through an 80-mesh sieve for later use.
[0033] (2) The starch is made into 4% starch slurry for subsequent use.
[0034] (3) Weigh the prescribed amount of stavudine, lactose, and microcrystalline cellulose, and place them in a fluidized granulator. Turn on the fan to make the material in a state suitable for fluidization, and spray starch slurry into it for fluidization granulation.
[0035] (4) Add the prescribed amount of hypromellose and magnesium stearate to the dry granules, sieve through a 20-mesh sieve, and compress into tablets.
[0036] (5) After passing the inspection of the ta...
Embodiment 2
[0048] 1. Tablet core prescription Raw material Feeding amount Weight ratio
[0049] Stavudine 50g 1
[0050]
[0051] Makes 1000 pieces
[0052]2. Tablet core preparation process: the specific steps are as in 2 in Example 1.
[0053] 3. Coating prescription
[0054] Raw material Feed amount Weight ratio
[0055] Hypromellose 4.7g 1
[0056] Ethylcellulose 8.46g 1.80
[0057] Dibutyl sebacate 1.64g 0.35
[0058] 85% ethanol 176g 37.4
[0059] 4. Coating process: the specific steps are as in 4 in Example 1.
Embodiment 3
[0061] 1. Tablet core prescription Raw material Feeding amount Weight ratio
[0062] Stavudine 50g 1
[0063]
[0064] Makes 1000 pieces
[0065] 2. Tablet core preparation process
[0066] The specific steps are as in 2 in Example 1.
[0067] 3. Coating prescription
[0068] Raw material Feed amount Weight ratio
[0069] Hypromellose 4.7g 1
[0070] Ethylcellulose 10.34g 2.2
[0071] Dibutyl sebacate 1.88g 0.4
[0072] 85% ethanol 188g 40
[0073] 4. Coating process
[0074] The specific steps are as in 4 in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com