Levamlodipine besylate tablet, preparation process thereof and control method for relevant materials
A technology of levamlodipine besylate and levorotatory besylate, which is applied in the field of levamlodipine besylate tablets and its preparation and related substances, and new dosage forms of levamlodipine, which can solve the problem of stability problems. To achieve the ideal and other problems, to achieve the effect of accelerated release speed, good therapeutic effect and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
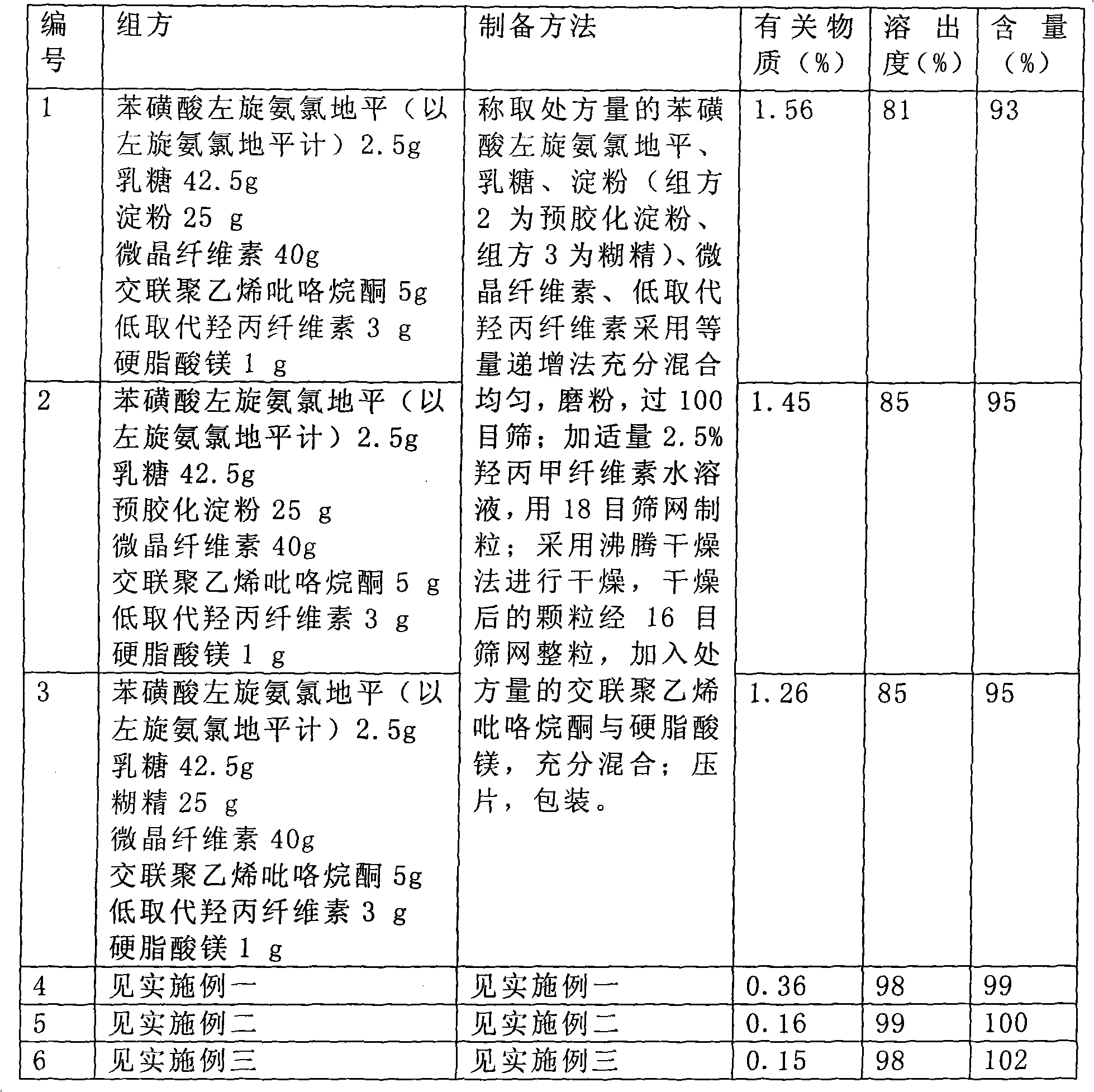
[0036] Embodiment one: levamlodipine besylate tablet
[0037] A kind of levamlodipine besylate tablet described in the present embodiment, every 1000 contains following components:
[0038] Levoamlodipine besylate 2.5g, calculated as levamlodipine;
[0039] Lactose 45.5g as filler;
[0040] β-cyclodextrin 20g, as inclusion agent;
[0041] Microcrystalline cellulose 45g, as a disintegrant;
[0042]Cross-linked polyvinylpyrrolidone 5g, as a disintegrant;
[0043] Magnesium stearate 1g, as a lubricant;
[0044] 2.5% hypromellose-50% ethanol 50g, as a binder.
[0045] The preparation method of the levamlodipine besylate sheet described in the present embodiment may further comprise the steps:
[0046] A) Weigh levamlodipine besylate and β-cyclodextrin according to the component content of 1000 tablets, add 2 to 5 times the amount of purified water to β-cyclodextrin and grind evenly, and mix levamlodipine besylate Soluble in 95% ethanol, fully mix the dissolved β-cyclodextri...
Embodiment 2
[0054] Embodiment two: levamlodipine besylate tablet
[0055] A kind of levamlodipine besylate tablet described in the present embodiment, every 1000 contains following components:
[0056] Levoamlodipine besylate 2.5g, calculated as levamlodipine;
[0057] Lactose 30g, as filler;
[0058] β-cyclodextrin 40g, as inclusion agent;
[0059] Microcrystalline cellulose 30g, as a disintegrant;
[0060] Cross-linked polyvinylpyrrolidone 15g, as a disintegrant;
[0061] Magnesium stearate 1.5g, as a lubricant;
[0062] 2.5% hypromellose-50% ethanol 60g, as a binder.
[0063] The preparation method of this embodiment is the same as that of Embodiment 1.
Embodiment 3
[0064] Embodiment Three: Levoamlodipine Besylate Tablets
[0065] A kind of levamlodipine besylate tablet described in the present embodiment, every 1000 contains following components:
[0066] Levoamlodipine besylate 2.5g, calculated as levamlodipine;
[0067] Lactose 50g, as filler;
[0068] β-cyclodextrin 29.5g, as inclusion agent;
[0069] Microcrystalline cellulose 25g, as a disintegrant;
[0070] Cross-linked polyvinylpyrrolidone 10g, as a disintegrant;
[0071] Magnesium stearate 2g, as a lubricant;
[0072] 2.5% hypromellose-50% ethanol 80g, as a binder.
[0073] The preparation method of this embodiment is the same as that of Embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com