Rizatriptan benzoate capsule and preparation method thereof
A technology of rizatriptan benzoate and triptan capsules, which is applied in the field of rizatriptan benzoate capsules and its preparation, can solve the problems of no obvious activity and low affinity, and achieve good dissolution rate and long storage life. Long, portable and easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
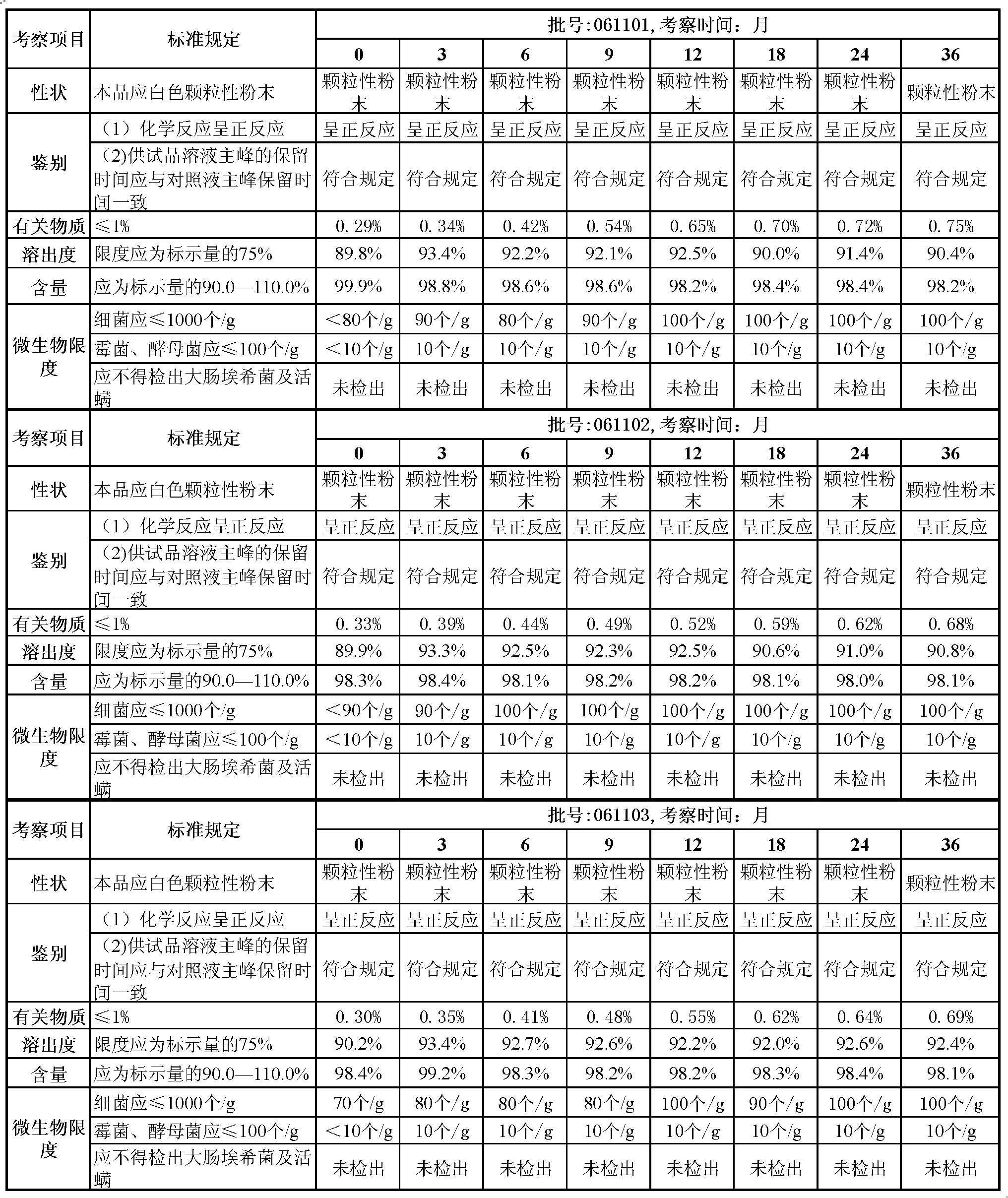
[0034] A kind of rizatriptan benzoate capsule (specification: 5mg / grain, calculated by rizatriptan), the formula that every 10000 capsules needs to be prepared is as follows:
[0035] Rizatriptan Benzoate
72.65g
200g
670g
15% polyvinylpyrrolidone ethanol solution
250ml
8g
[0036] The preparation method of rizatriptan benzoate capsule, comprises the steps:
[0037] Step 1: respectively pulverize rizatriptan benzoate, microcrystalline cellulose, starch and magnesium stearate through a 100-mesh sieve, and set aside;
[0038] Step 2: Weigh the raw material rizatriptan benzoate and auxiliary materials microcrystalline cellulose, starch and magnesium stearate according to the above composition formula;
[0039] Step 3: Preparation of adhesive: Add polyvinylpyrrolidone to 80 to 98% ethanol, stir to dissolve completely, and prepare a 15% polyvinylpyrrolidone eth...
Embodiment 2
[0046] A kind of rizatriptan benzoate capsule (specification: 5mg / grain, calculated according to rizatriptan.), the formula that every 10000 capsules required for preparing is as follows:
[0047] Rizatriptan Benzoate
72.65g
300g
starch
700g
15% polyvinylpyrrolidone ethanol solution
300ml
Magnesium stearate
10g
[0048] The preparation method of rizatriptan benzoate capsule, comprises the steps:
[0049] Step 1: respectively pulverize rizatriptan benzoate, microcrystalline cellulose, starch and magnesium stearate through a 100-mesh sieve, and set aside;
[0050] Step 2: Weigh the raw material rizatriptan benzoate and auxiliary materials microcrystalline cellulose, starch and magnesium stearate according to the above composition formula;
[0051] Step 3: Prepare adhesive: add 45g of polyvinylpyrrolidone to 95% ethanol to make 300ml, stir to dissolve completely, and prepare a 15% po...
Embodiment 3
[0058] A kind of rizatriptan benzoate capsule (specification: 10mg / grain, calculated according to rizatriptan.), the formula that every preparation 10000 capsules needs is as follows:
[0059] Rizatriptan Benzoate
145.3g
microcrystalline cellulose
400g
starch
800g
[0060] 15% polyvinylpyrrolidone ethanol solution
500ml
Magnesium stearate
8g
[0061] The preparation method of rizatriptan benzoate capsule, comprises the steps:
[0062] Step 1: respectively pulverize rizatriptan benzoate, microcrystalline cellulose, starch and magnesium stearate through a 100-mesh sieve, and set aside;
[0063] Step 2: Weigh the raw material rizatriptan benzoate and auxiliary materials microcrystalline cellulose, starch and magnesium stearate according to the above composition formula;
[0064] Step 3: Prepare adhesive: add 75g of polyvinylpyrrolidone to 95% ethanol to make 500ml, stir to dissolve completely, and pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com