Metformin hydrochloride enteric-coated tablets and preparation method thereof
A metformin hydrochloride enteric and metformin hydrochloride technology are applied in the field of metformin hydrochloride enteric-coated tablets and the preparation thereof, and can solve the problems such as the need for further improvement, and achieve the effects of reducing the increase of related substances and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
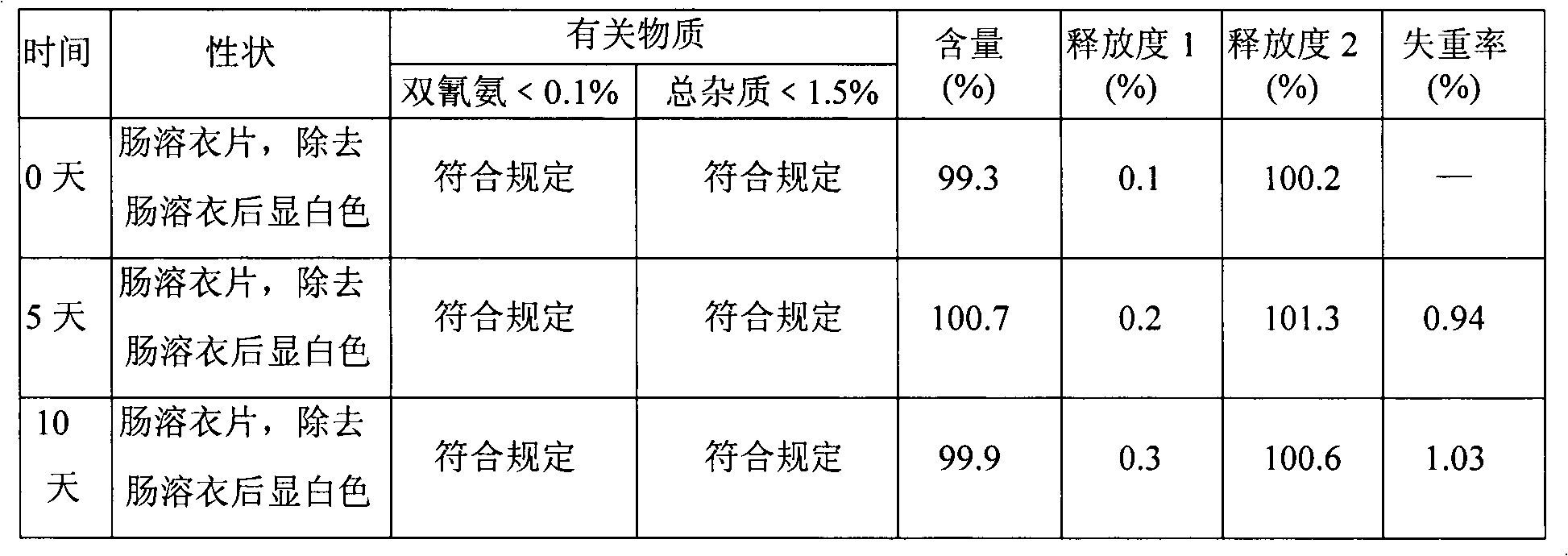
[0022] Embodiment 1. Metformin hydrochloride enteric-coated tablets
[0023] Tablet core prescription (10000 tablets)
[0024] Name of raw material Amount (g)
[0025] Metformin Hydrochloride 2500
[0026] Dextrin 500
[0027] Hypromellose 30 (additional)
[0028] Hypromellose 30 (additional)
[0031] 4% hypromellose 15% ethanol aqueous solution appropriate amount
[0032] Isolation gown coating solution prescription
[0033] Raw material name dosage
[0034] Opadry (stomach-soluble film-coated pre-suspension) 90g
[0035] Purified water 500g
[0036] Enteric Coating Solution Prescription
[0037] Raw material name dosage
[0038] Opadry (enteric-coated film-coated pre-suspension) 300g
[0039] Purified water 1200g
[0040] Preparation:
[0041] 1. The raw materials are crushed and passed through an 80-mesh sieve, and the dextrin, hydroxypropyl cellulose, magnesium stearate, and talcum powder are passed thro...
Embodiment 2
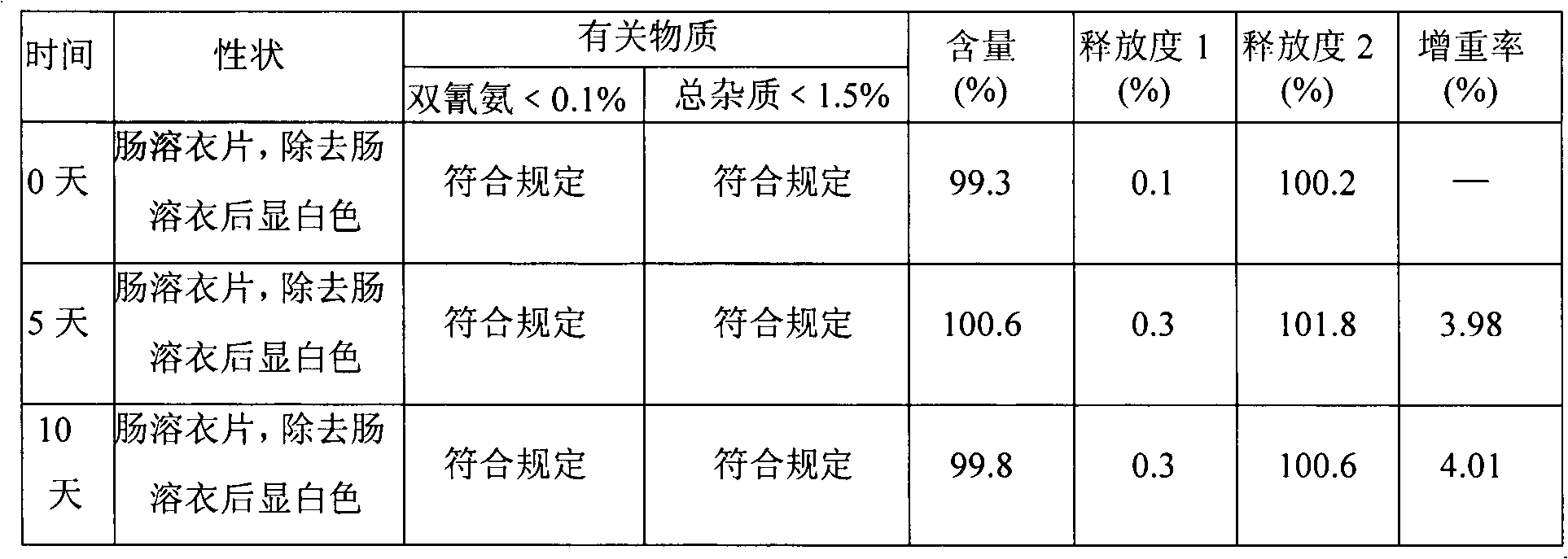
[0050] Embodiment 2. Metformin hydrochloride enteric-coated tablets
[0051] Tablet core prescription (10000 tablets)
[0052] Name of raw material Amount (g)
[0053] Metformin Hydrochloride 2500
[0054] Dextrin 450
[0055] Hypromellose 25 (additional)
[0056]Hypromellose 25 (additional)
[0059] 4% hypromellose 15% ethanol aqueous solution appropriate amount
[0060] Isolation gown coating solution prescription
[0061] Raw material name dosage
[0062] Opadry (stomach-soluble film-coated pre-suspension) 85g
[0063] Purified water 450g
[0064] Enteric Coating Solution Prescription
[0065] Raw material name dosage
[0066] Opadry (enteric-coated film-coated pre-suspension) 250g
[0067] Purified water 1100g
[0068] Prepare with reference to embodiment 1 method
Embodiment 3
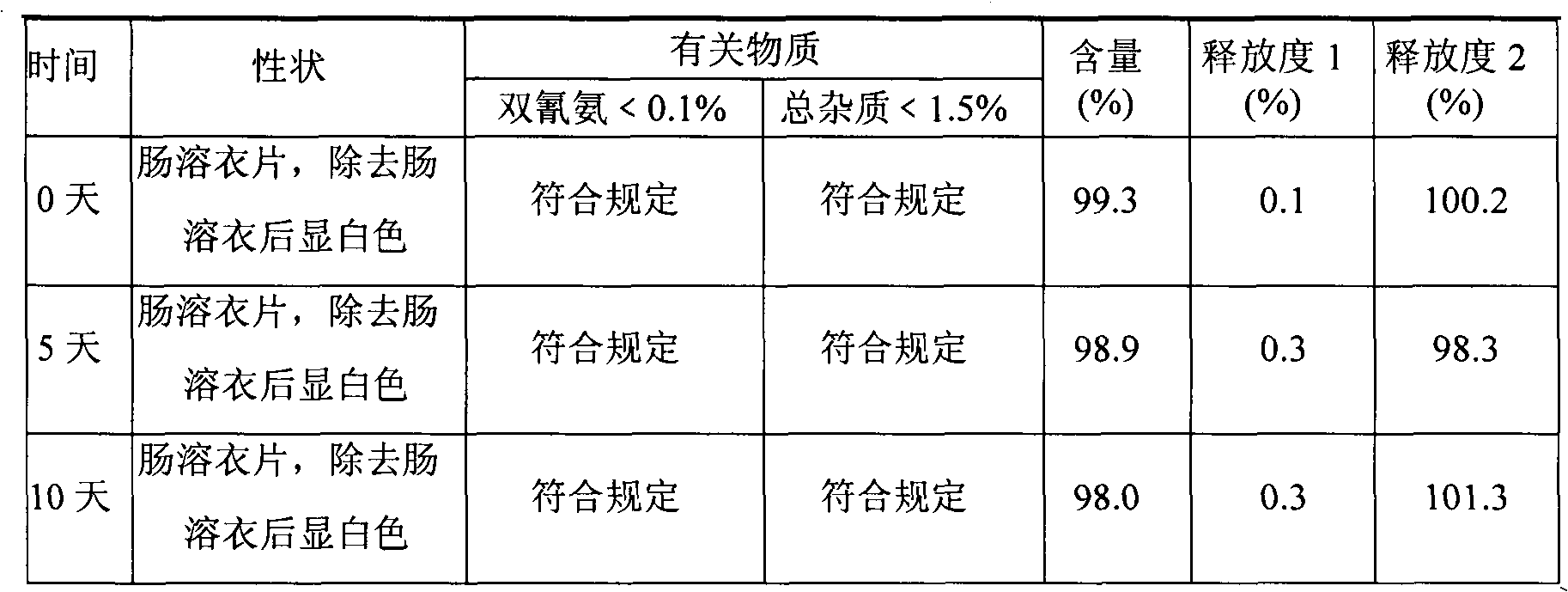
[0069] Embodiment 3. Metformin hydrochloride enteric-coated tablets
[0070] Tablet core prescription (10000 tablets)
[0071] Name of raw material Amount (g)
[0072] Metformin Hydrochloride 2500
[0073] Dextrin 550
[0074] Hypromellose 35 (additional)
[0075] Hypromellose 35 (additional)
[0077] Talc 305
[0078] 4% hypromellose 15% ethanol aqueous solution appropriate amount
[0079] Isolation gown coating solution prescription
[0080] Raw material name dosage
[0081] Opadry (stomach-soluble film-coated pre-suspension) 95g
[0082] Purified water 550g
[0083] Enteric Coating Solution Prescription
[0084] Raw material name dosage
[0085] Opadry (enteric-coated film-coated pre-suspension) 350g
[0086] Purified water 1300g
[0087] Prepare with reference to embodiment 1 method
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com