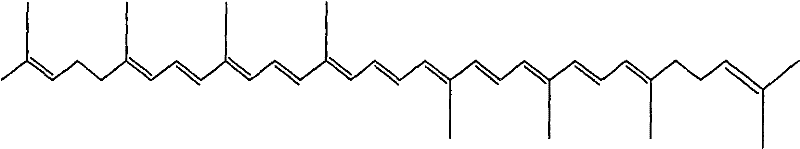
Enteric solid preparation containing lycopene, resveratrol or melatonin and preparation method of enteric solid preparation
A technology of solid preparations and lycopene, which is applied in the field of enteric-coated solid preparations and its preparation, and can solve problems such as poor solubility improvement, loose binding, and unsaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation method of the water-soluble / enteric-coated solid dispersion of the present invention can adopt the commonly used method for preparing solid dispersion, including melting method, solvent method, solvent-melting method, grinding method, solvent-freeze drying method, solvent-spray drying method and hot-melt extrusion method, etc., for details, please refer to pages 26-29 of the first edition of "New Dosage Forms of Drugs" published by Chemical Industry Press and edited by Zhu Shengshan.
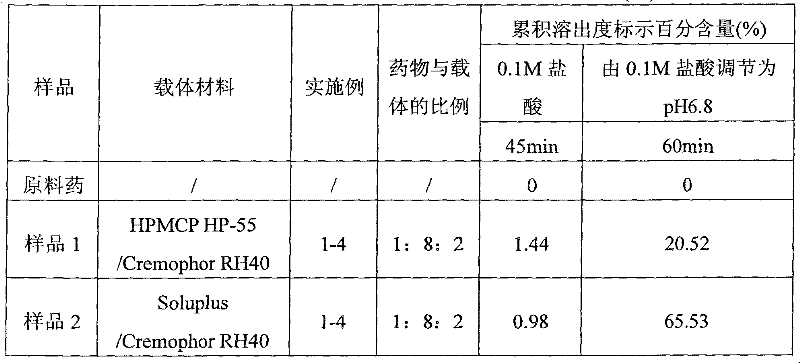
[0062] As a preferred embodiment, the present invention provides an enteric solid preparation, which contains lycopene as an active ingredient, an enteric carrier and / or a water-soluble carrier; The solid dispersion form exists in the preparation, or the water-soluble carrier and the lycopene exist in the solid preparation in the form of a water-soluble solid dispersion, and the solid preparation is coated with an enteric-coated carrier containing an enteric carrier. coati...
Embodiment 1-1
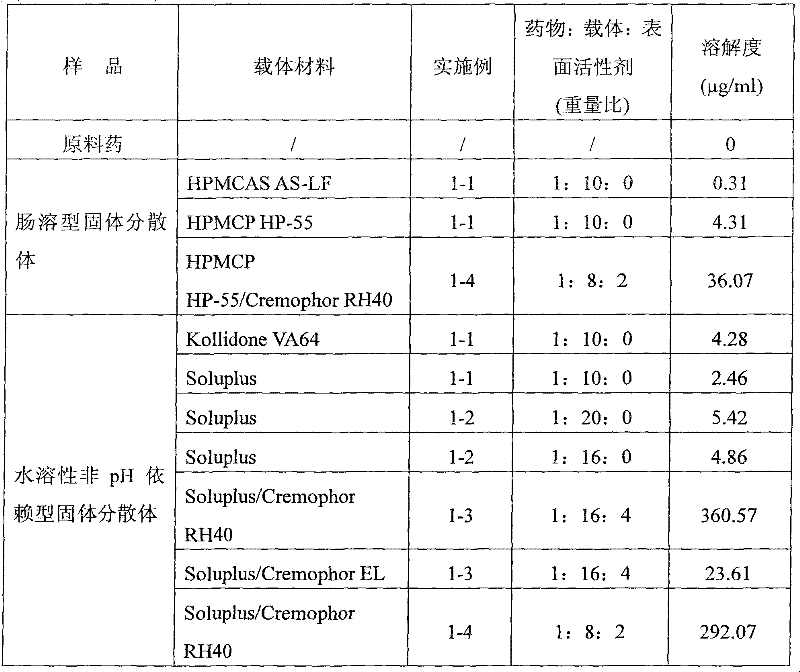
[0094] Weigh 10g each of HPMCAS AS-LF, HPMCP HP-55, Kollidone VA64, Poloxamer188, and Soluplus and dissolve them in 1000g of dichloromethane, then add 0.05g of vitamin C to the above solution and stir until completely dissolved, then add lycopene respectively (manufactured by Changsha Huirui Biotechnology Co., Ltd.) 1 g to form a homogeneous solution. Use Buchi mini spray dryer B-290 to spray dry to remove the organic solvent to obtain a solid dispersion of lycopene. During the spray drying process, the temperature of the material is controlled at 50-55°C.
Embodiment 1-2
[0096] Weigh 20g and 16g of Soluplus and dissolve them in 1000g of dichloromethane respectively, then add 0.05g of vitamin C to the above solution and stir until completely dissolved, then add 1g of lycopene (produced by Changsha Huirui Biotechnology Co., Ltd.) to form homogeneous solution. Use Buchi mini spray dryer B-290 to spray dry to remove the organic solvent to obtain a solid dispersion of lycopene. During the spray drying process, the temperature of the material is controlled at 50-55°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com