Preparation process for ultrafine glibenclamide particles
A fine-grained and granular technology, which is applied to the active ingredients of sulfonylureas, devices that make medicines into special physical or taking forms, and metabolic diseases, etc. To achieve the effect of easy transportation and storage, good stability, easy to scale up and large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A: Weigh 0.5g glibenclamide bulk drug and dissolve it in 10ml DMF;
[0039] B: Weigh 0.5g of hydroxypropyl methylcellulose and 0.018g of sodium dodecylbenzenesulfonate and dissolve them in 300mL of water, put the beaker containing the aqueous solution in an ice-water bath, and control the temperature of the aqueous solution at about 2°C;
[0040] C: under the stirring condition of 2000rpm, pour the bulk drug solution prepared in step A into the aqueous solution of step B to obtain drug slurry;
[0041] D: the inlet temperature of the control spray dryer (SD-Basic, Labplant, UK) is 160°C, the outlet temperature is 83°C, the feed rate is 28ml / min, the compressed air pressure is 0.6MPa, and the drug slurry is spray-dried, and then The superfine glibenclamide drug composite powder is obtained.
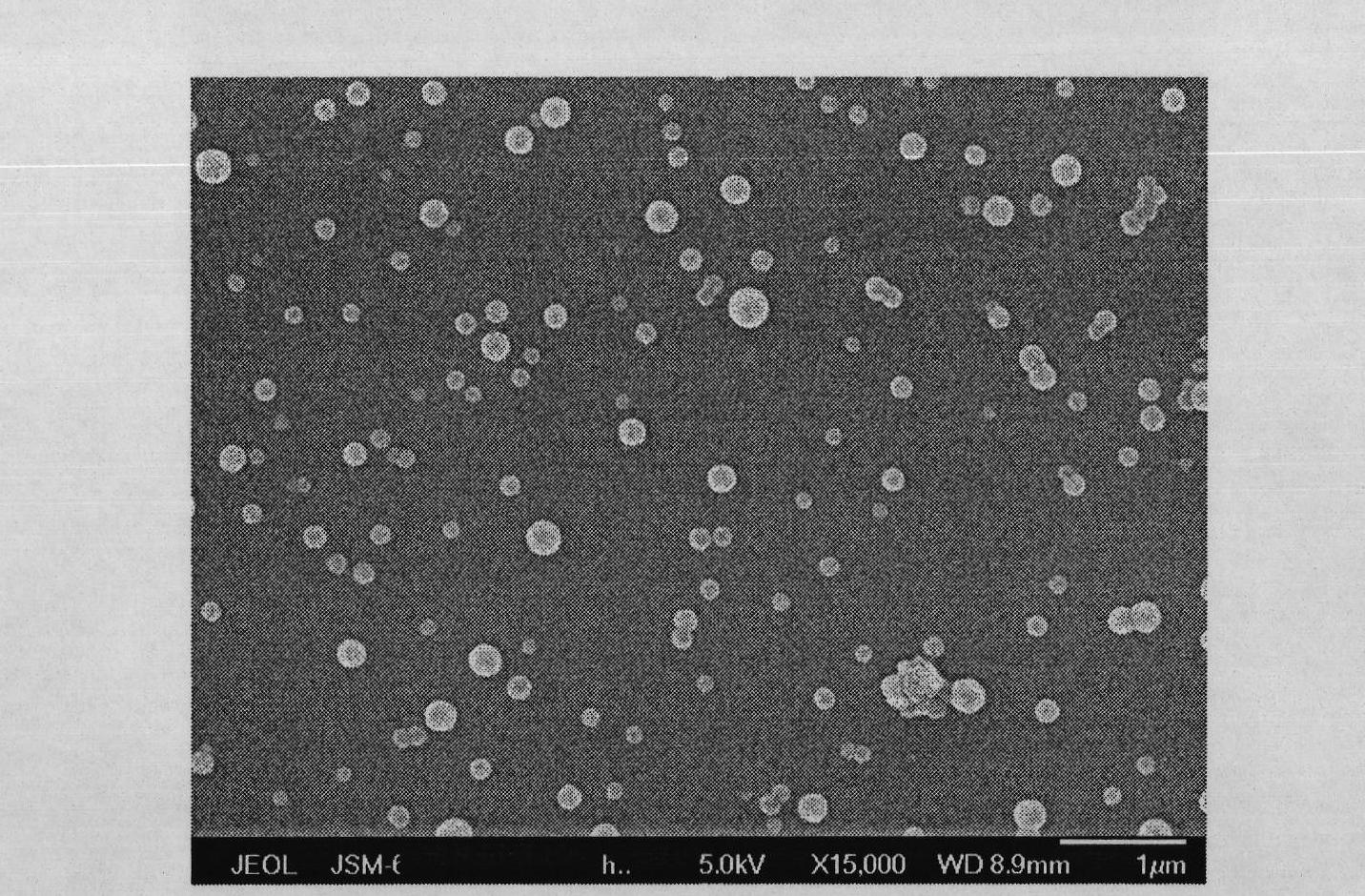
[0042] figure 1 It is the scanning electron micrograph of glibenclamide API; figure 2 It is the scanning electron micrograph of the particles in the glibenclamide slurry of embo...
Embodiment 2
[0044] A: Weigh 0.5g glibenclamide bulk drug and dissolve it in 10ml DMF;
[0045] B: Weigh 0.5g of hydroxypropyl methylcellulose and dissolve it in 300mL of water, bathe the beaker containing the aqueous solution in ice water, and control the temperature of the aqueous solution at about 2°C;
[0046] C: under the stirring condition of 2000rpm, pour the bulk drug solution prepared in step A into the aqueous solution of step B to obtain drug slurry;
[0047] D: the inlet temperature of the control spray dryer (SD-Basic, Labplant, UK) is 160°C, the outlet temperature is 83°C, the feed rate is 28ml / min, the compressed air pressure is 0.6MPa, and the drug slurry is spray-dried, and then The superfine glibenclamide drug composite powder is obtained.
Embodiment 3
[0049] A: Weigh 4g glibenclamide bulk drug and dissolve it in 80ml DMF;
[0050] B: Weigh 4g of hydroxypropyl methylcellulose and 0.14g of sodium dodecylbenzenesulfonate and dissolve them in 2400mL of water, bathe the beaker containing the aqueous solution in ice water, and control the temperature of the aqueous solution at about 2°C;
[0051] C: under the stirring condition of 2000rpm, pour the bulk drug solution prepared in step A into the aqueous solution of step B to obtain drug slurry;
[0052] D: the inlet temperature of the control spray dryer (SD-Basic, Labplant, UK) is 160°C, the outlet temperature is 83°C, the feed rate is 28ml / min, the compressed air pressure is 0.6MPa, and the drug slurry is spray-dried, and then The superfine glibenclamide drug composite powder is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com