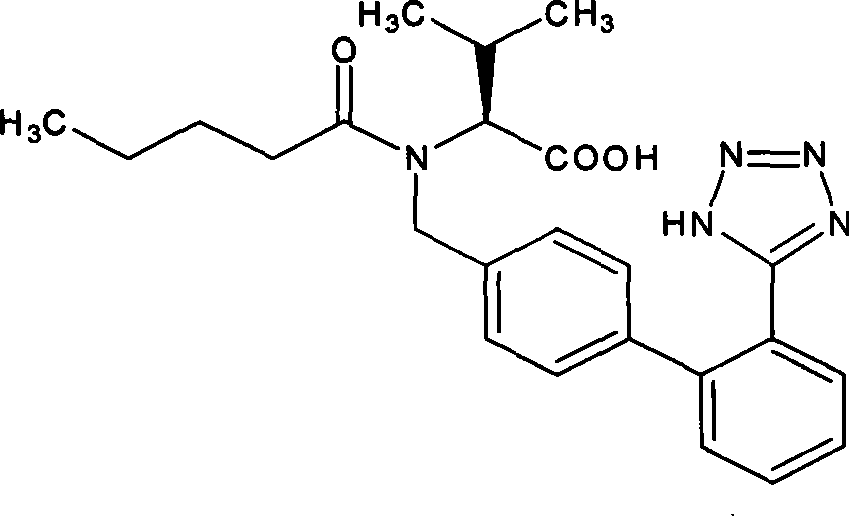
Diovan compound preparation and preparation method thereof
A compound preparation and valsartan technology, applied in the field of pharmaceutical preparations and valsartan compound preparations, can solve problems such as being unsuitable for industrial production, poor powder fluidity, poor material fluidity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: the preparation of valsartan amlodipine tablet
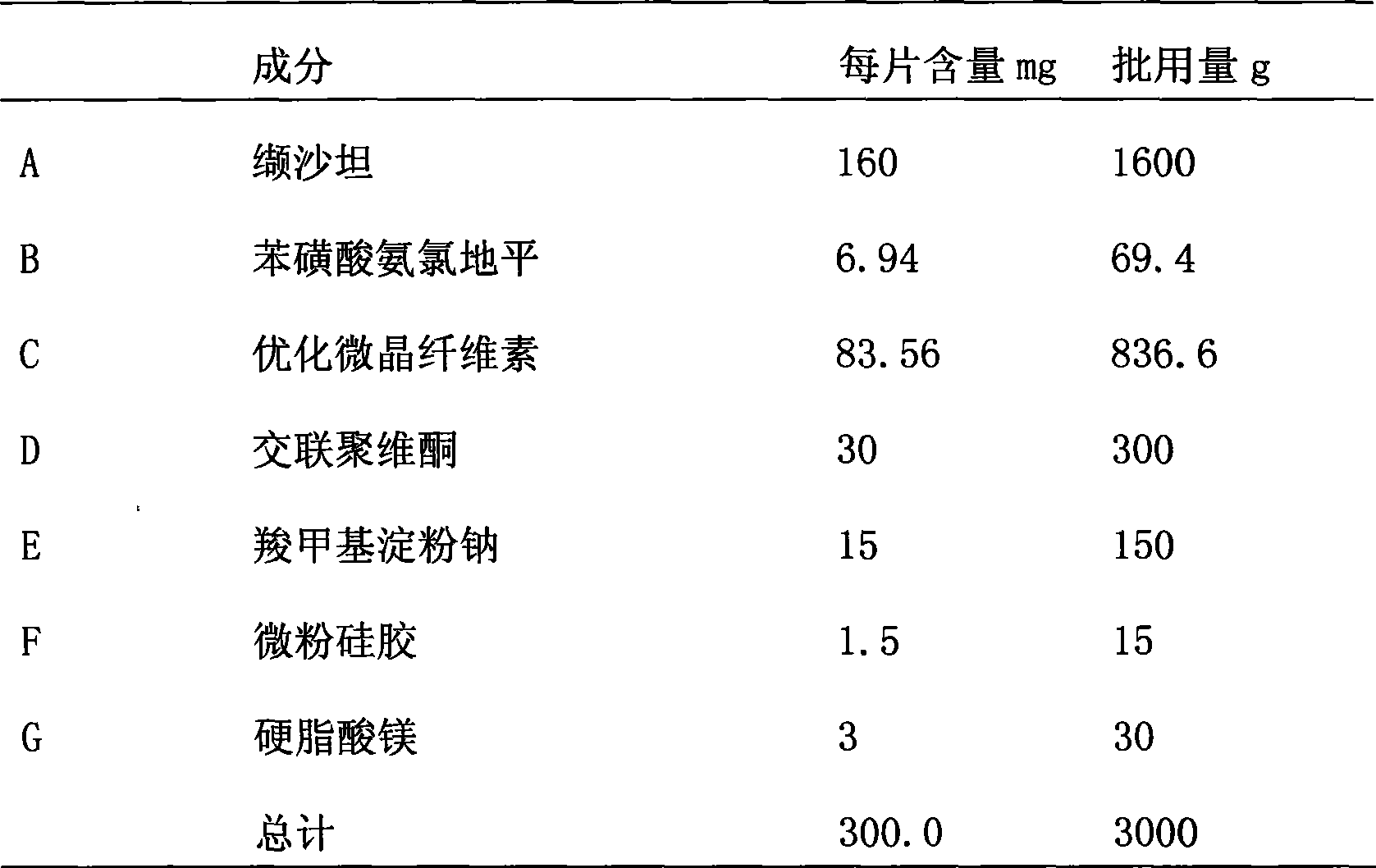
[0078] The material composition is shown in Table 1;
[0079] Table 1. Ratio of material composition
[0080]
[0081] Preparation of tablet cores: the whole valsartan bulk drug of the prescribed amount is granulated by a dry granulator (Alexander WP120V) to obtain valsartan bulk drug granules. Pressure: 40bar, roller speed: 3rpm. The granules are mixed with amlodipine besylate in the prescribed amount, optimized microcrystalline cellulose, crospovidone, sodium carboxymethyl starch, micropowder silica gel, and magnesium stearate, and then compressed by a high-speed tablet machine.
[0082] Preparation of coated tablets: the coating powder is formulated into a coating solution, and the plain tablets are coated. Until the weight gain of the tablet is 2-5%, all indexes of the coated tablet meet the relevant regulations. The results are shown in Table 2.
[0083] Table 2. Test results of valsartan and am...
Embodiment 2
[0085] Embodiment 2: the preparation of valsartan amlodipine tablet
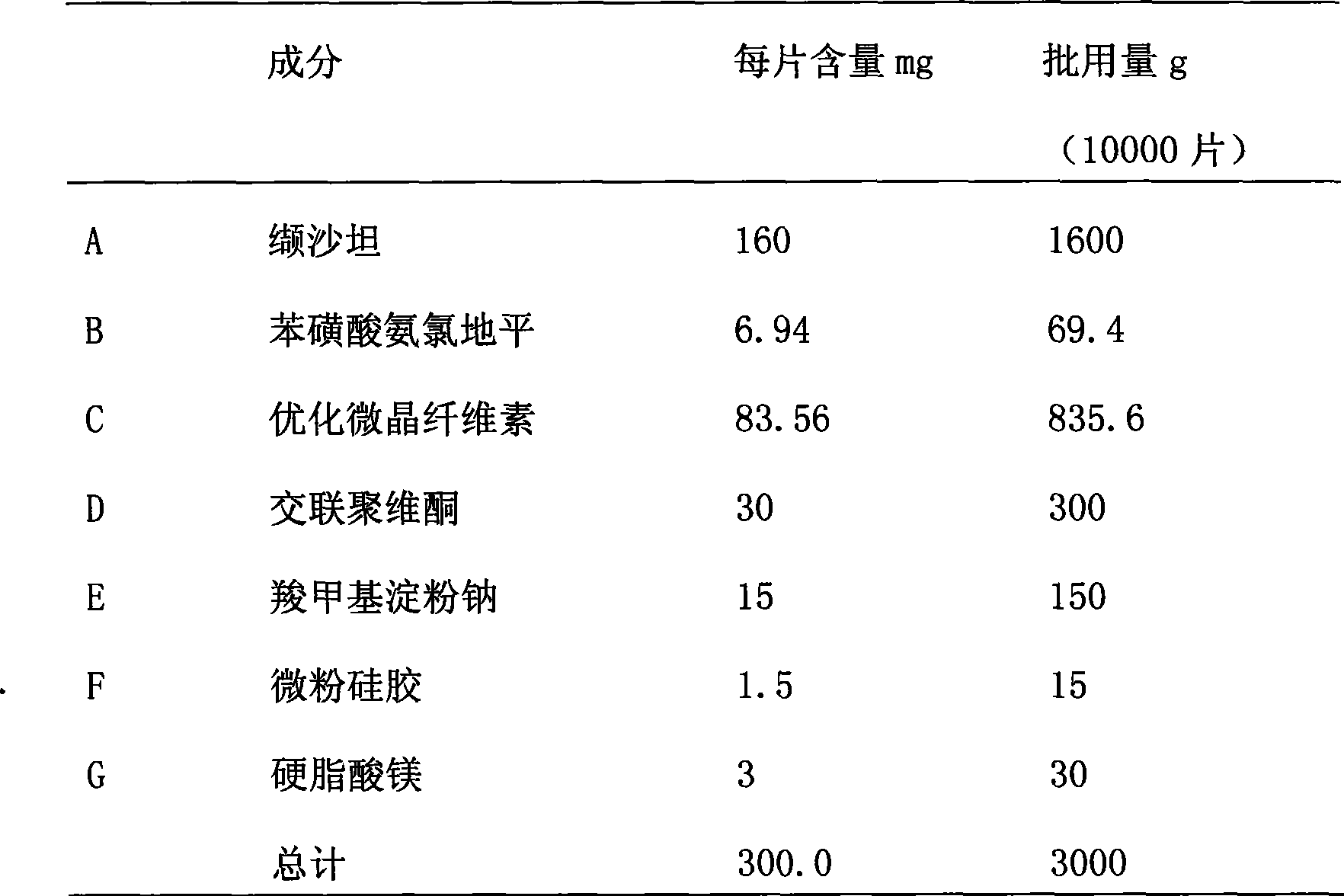
[0086] The material composition is shown in Table 3;
[0087] Preparation of tablet cores: the whole valsartan bulk drug of the prescribed amount is granulated by a dry granulator (Alexander WP120V) to obtain valsartan bulk drug granules. Pressure: 40bar, roller speed: 3rpm. The granules are mixed with amlodipine besylate in the prescribed amount, optimized microcrystalline cellulose, crospovidone, sodium carboxymethyl starch, micropowder silica gel, and magnesium stearate, and then compressed by a high-speed tablet machine.
[0088] Table 3. Ratio of material composition
[0089]
[0090]
[0091] Preparation of coated tablets: the coating powder is formulated into a coating solution, and the plain tablets are coated. Until the weight gain of the tablet is 2-5%, all indexes of the coated tablet meet the relevant regulations, and the results are shown in Table 4.
[0092] Table 4. Test results of v...
Embodiment 3
[0094] Embodiment 3: the preparation of valsartan amlodipine capsule
[0095] The material composition is shown in Table 3;
[0096] Preparation steps: use a dry granulator (Alexander WP120V) to granulate all the valsartan raw materials in the prescription to obtain valsartan raw drug granules. Pressure: 40bar, roller speed: 3rpm. The granule is mixed with amlodipine besylate of the prescribed amount, optimized microcrystalline cellulose, crospovidone, sodium carboxymethyl starch, micropowder silica gel, and magnesium stearate, and then capsule filling is performed by a capsule filling machine. And the weight difference, disintegration time limit and dissolution of the capsules were detected. The results are shown in Table 5.
[0097] Table 5 Test results of valsartan and amlodipine capsules
[0098]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com