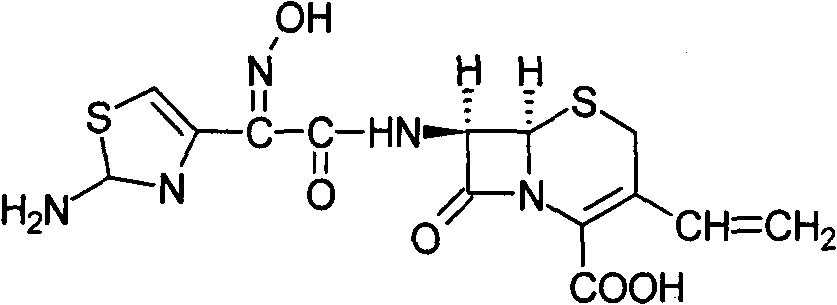
Cefdinir dispersible tablet and preparation method thereof
A technology for cefdinir and dispersible tablets, applied in the field of cefdinir dispersible tablets and their preparation, can solve the problems of fluctuating clinical efficacy of tablets, slow disintegration and dissolution, low bioavailability and the like, and achieve continuous blood drug concentration. Good properties, increased drug distribution area, and low drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
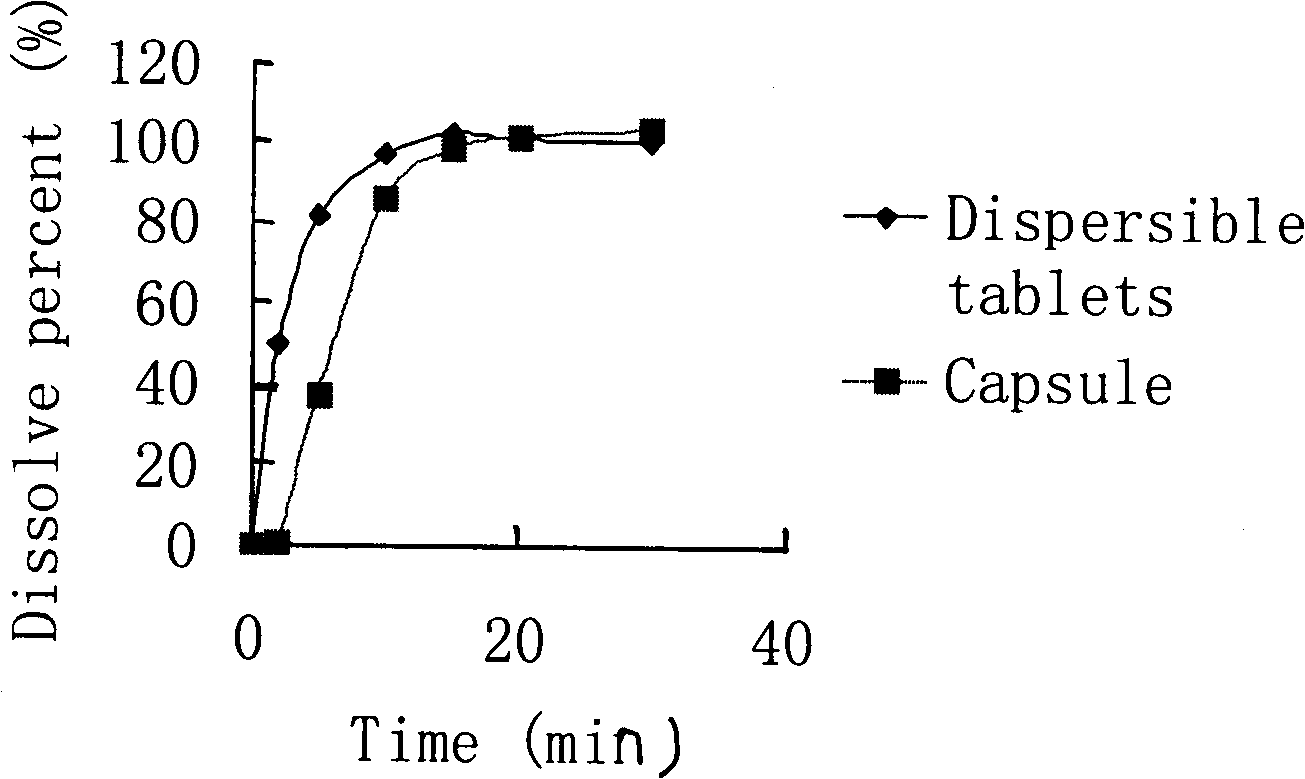
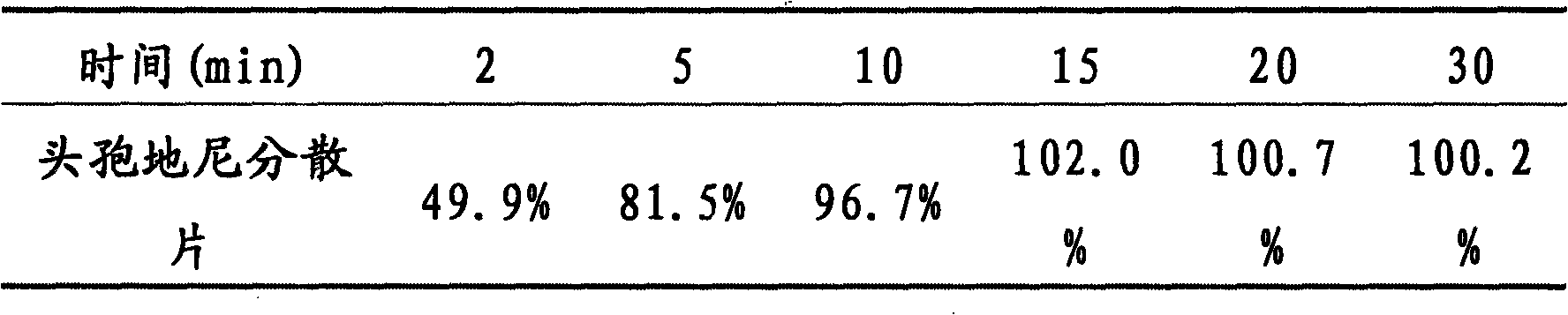
Image
Examples
Embodiment 1
[0043] Specification: 50mg
[0044] Product name Quantity per piece
[0045] Cefdinir 50.00mg
[0046] Crospovidone (PVPP) 31.25mg
[0047] Microcrystalline Cellulose (MCC) 62.50mg
[0048] Aspartame 0.25mg
[0049] Micronized silica gel 3.00mg
[0050] Magnesium Stearate 1.50mg
[0051] Total: 148.50mg
Embodiment 2
[0053] Specification: 0.1g
[0054] Product name Quantity per piece
[0055] Cefdinir 100.00mg
[0056] Crospovidone (PVPP) 62.50mg
[0057] Microcrystalline Cellulose (MCC) 125.00mg
[0058] Aspartame 0.50mg
[0059] Micronized silica gel 6.00mg
[0060] Magnesium Stearate 3.00mg
[0061] Total: 297mg
Embodiment 3
[0063] Specification: 50mg
[0064] Product name Quantity per piece
[0065] Cefdinir 50.00mg
[0066] Crospovidone (PVPP) 31.25mg
[0067] Microcrystalline Cellulose (MCC) 62.50mg
[0068] Aspartame 0.25mg
[0069] Micronized silica gel 3.00mg
[0070] Magnesium Stearate 1.50mg
[0071] Total: 148.50mg
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com