Application of silicon dioxide aerogel in pharmacy
A technology of silica and airgel, applied in the fields of silica, silicon oxide, inorganic chemistry, etc., can solve the problems of nano-drug loading systems that have not yet been discovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Preparation of paclitaxel nanoparticles:
[0117] 1. Paclitaxel API (Yunnan Hande Pharmaceutical Co., Ltd.) 1g, add 20ml of absolute ethanol to dissolve;
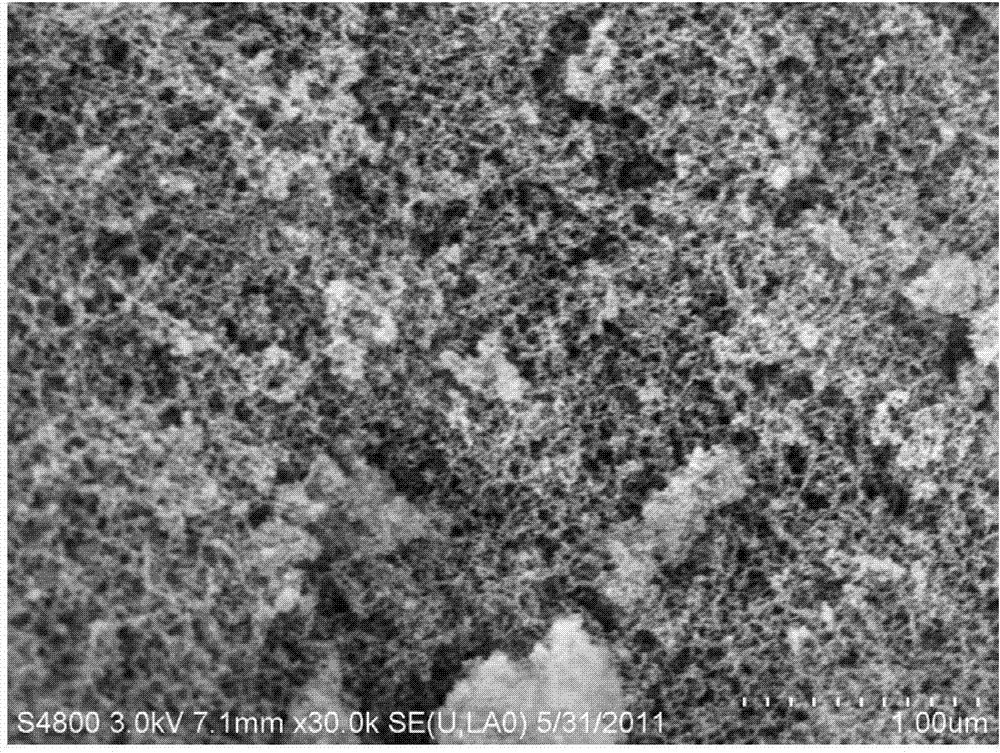
[0118] 2. Add silica airgel after heat treatment at 300°C (porosity 95%, pore diameter 10nm, specific surface area 1000m 2 / g, the density is 300kg / m 3 , The diameter of the colloidal particles that make up the network is 20nm) 2g for adsorption;
[0119] 3. After the adsorption is complete, dry it in an oven at 60°C;
[0120] 4. After drying, add 100ml of pure water and emulsify with 25000rpm / min ordinary emulsifier for 5min;
[0121] 5. High-pressure homogenizer (Shanghai Donghua GYB30-6S), 400bar, cycle 6 times, 10min;
[0122] 6. Spray-dry the homogeneous liquid in an experimental spray dryer (Shanghai Shunyi Technology SP-1500), parameters: temperature 130°C, flow rate 500ml / H, nozzle: 0.75mm, and obtain paclitaxel nanoparticles after drying.
Embodiment 2
[0124] Preparation of paclitaxel nanoparticles:
[0125] 1. Paclitaxel API (Yunnan Hande Pharmaceutical Co., Ltd.) 1g, add 100ml of absolute ethanol to dissolve;
[0126] 2. Add hydrophilic silica airgel (porosity 97%, pore diameter 16nm, specific surface area 500m 2 / g, the density is 150kg / m 3 , The diameter of the colloidal particles that make up the network is 50nm) 10g for adsorption;
[0127] 3. After the adsorption is complete, freeze-dry;
[0128] 4. After drying, add 110ml of pure water and emulsify with 25000rpm / min ordinary emulsifier for 5min;
[0129] 5. High-pressure homogenizer (Shanghai Donghua GYB30-6S), 400bar, cycle 7 times, 10min;
[0130] 6. Spray-dry the homogeneous liquid in an experimental spray dryer (Shanghai Shunyi Technology SP-1500), parameters: temperature 130°C, flow rate 500ml / H, nozzle: 0.75mm, and obtain paclitaxel nanoparticles after drying.
Embodiment 3
[0132] Preparation of insulin nanoparticles:
[0133] 1. Add 150ml of 0.01mol / L AR grade hydrochloric acid to dissolve 1g of insulin raw material (Jiangsu Wanbang Biochemical Pharmaceutical Co., Ltd.);
[0134] 2. Add silica airgel after heat treatment at 1000℃ (porosity is 99%, pore diameter is 50nm, specific surface area is 500m 2 / g, the density is 3kg / m 3 , The diameter of the colloidal particles that make up the network is 1nm) 15g for adsorption;
[0135] 3. After complete adsorption, dry with a freeze dryer for 4 hours;
[0136]4. Add another 10g of PEG-600 to 1000ml of absolute ethanol to dissolve;
[0137] 5. Add the lyophilized solid in step 3 into the above ethanol solution of PEG-600, and emulsify with a ultrasonic emulsifier for 3 minutes;
[0138] 6. Dry the emulsion in step 5 in a 60°C electric constant temperature drying oven for 12 hours;
[0139] 7. Grinding the dried solid in step 6, and passing through a 200-mesh sieve to obtain insulin nanoparticles. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com