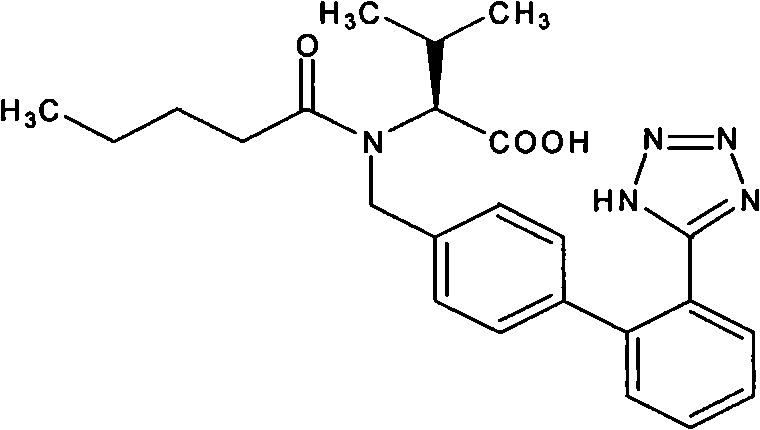
Valsartan-containing solid preparation and preparation method thereof
A technology of valsartan and preparations, which is applied in the field of pharmaceutical preparations and its preparation, and can solve problems such as inconvenience for patients to take, difficulty in tablet disintegration, and slow dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
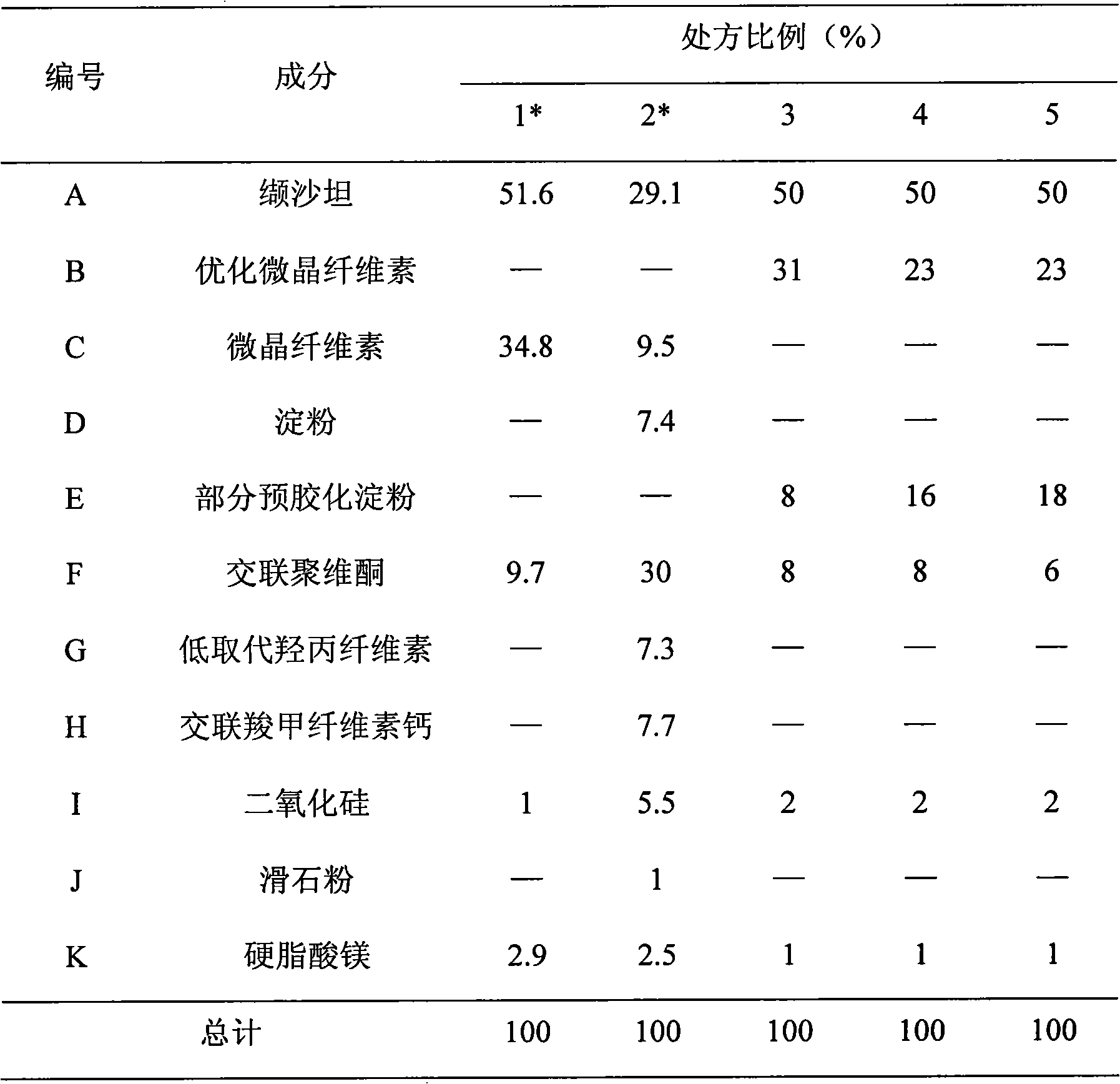
[0082] Embodiment 1: the preparation of valsartan tablet
[0083] The material composition is shown in Table 1;
[0084] Table 1 Material Composition Ratio
[0085]
[0086] *Prescriptions 1 and 2 are here only for comparison and do not belong to the content of the present invention. Prescription 1 comes from Example 7 of the patent application CN1636561A in the background technology, and prescription 2 comes from the prescription in Table 1 of the example of the patent application WO05089720 in the background technology 1.
[0087] Preparation of tablet cores: the prescribed amount of valsartan was granulated by roller compaction (Alexander WP120V), pressure: 40bar, roller speed: 3rpm. The prepared granules are mixed with other auxiliary materials, and then a punch with a suitable size is selected according to the weight of the tablet, and a high-speed tablet press is used to compress the tablet.
[0088] Preparation of coated tablets: Weigh The 85G series coating pow...
Embodiment 2
[0092] Embodiment 2: the preparation of valsartan tablet
[0093] The material composition is shown in Table 3.
[0094] Table 3 Material Composition Ratio
[0095]
[0096]
[0097] Preparation of tablet cores: the prescribed amount of valsartan was granulated by roller compaction (Alexander WP120V), pressure: 60bar, roller speed: 3rpm. Mix the prepared granules with optimized microcrystalline cellulose, crospovidone, low-substituted hydroxypropyl cellulose, and micropowder silica gel in a total mixing tank, then add magnesium stearate and mix evenly, and set aside. Use 14*5.5mm oval shallow concave punching plain tablet.
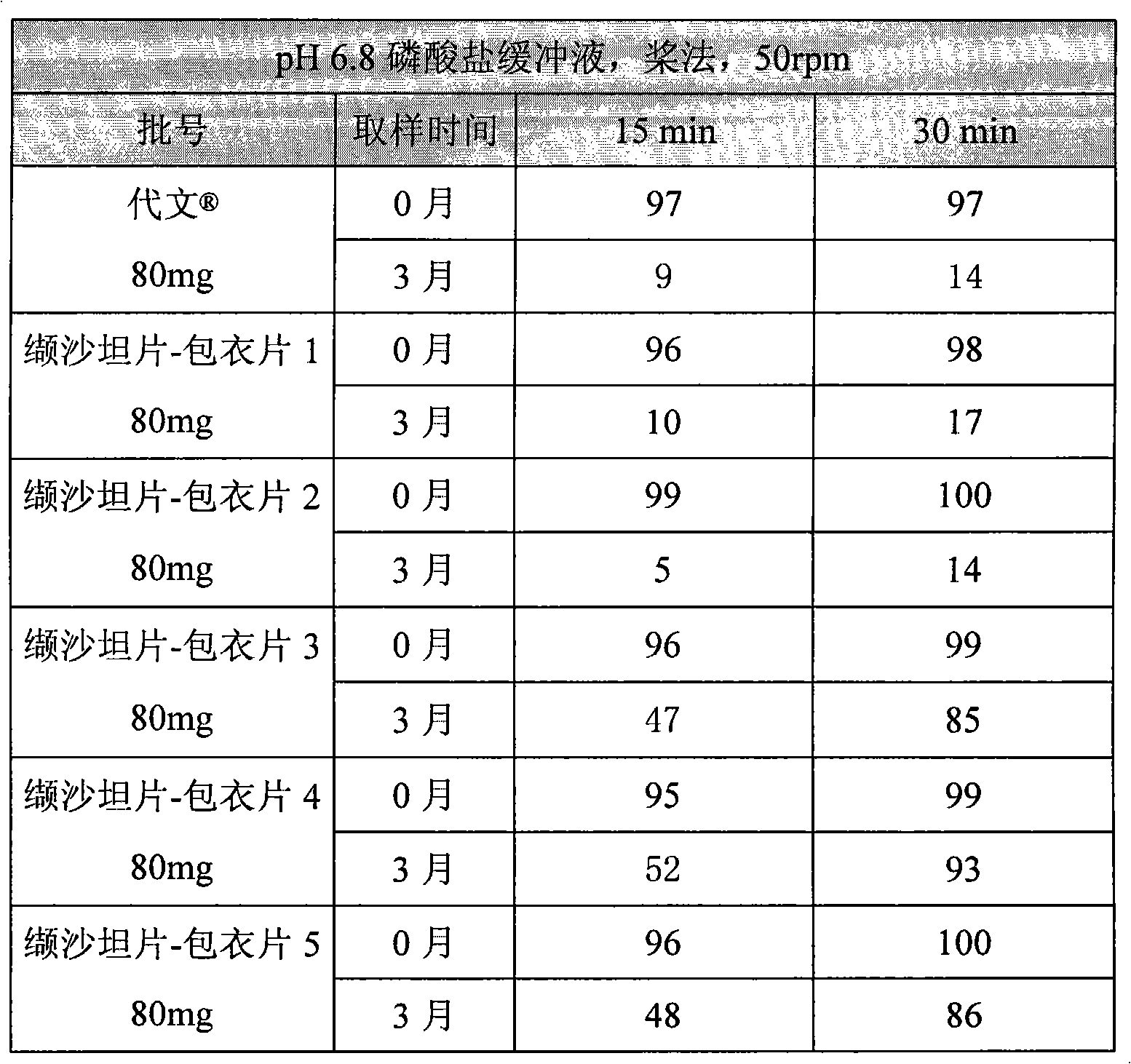
[0098] Preparation of coated tablets: Weigh 85G series coating powder prepares a coating liquid with a solid content of 20%, and coats plain tablets. Until the sheet has gained about 3% in weight. The indicators of the coated tablets meet the relevant regulations. The specific results are shown in Table 4.
[0099] Table 4 Test results of val...
Embodiment 3
[0101] Embodiment 3: the preparation of valsartan tablet
[0102] The material composition is shown in Table 5.
[0103] Table 5 Material Composition Ratio
[0104]
[0105]
[0106] Preparation of tablet cores: the prescribed amount of valsartan was granulated by roller compaction (Alexander WP120V), pressure: 70bar, roller speed: 3rpm. Mix the prepared granules with powdered cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium and silicon dioxide in a mixing tank, then add magnesium stearate and mix evenly, and set aside. A circular shallow concave stamping sheet with a diameter of φ8.0mm is used.
[0107] Preparation of coated tablets: Weigh A coating solution is prepared to coat the plain tablets. Until the sheet has gained about 3% in weight. The results are shown in Table 6.
[0108] Table 6 Test results of valsartan tablets
[0109]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com