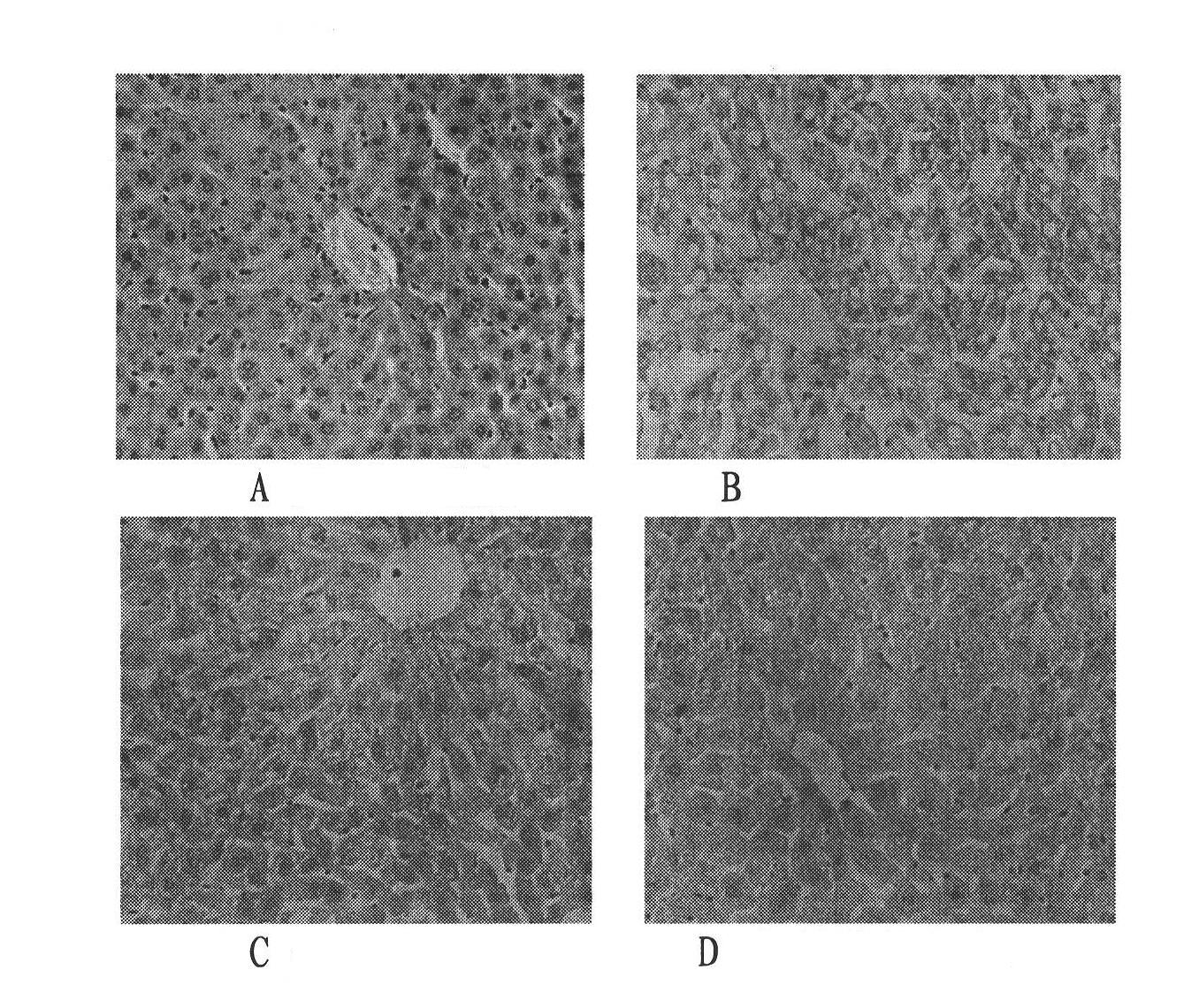
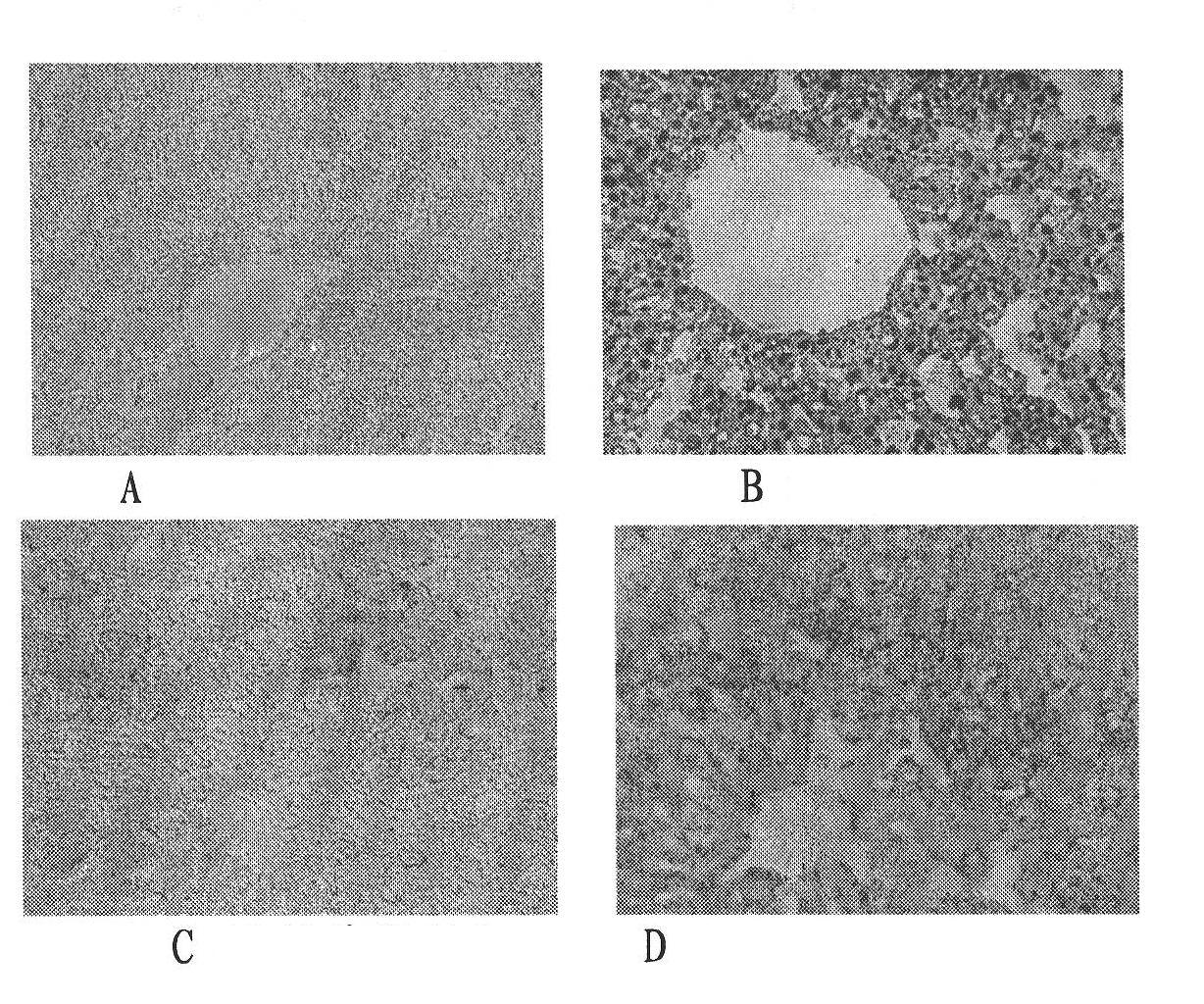
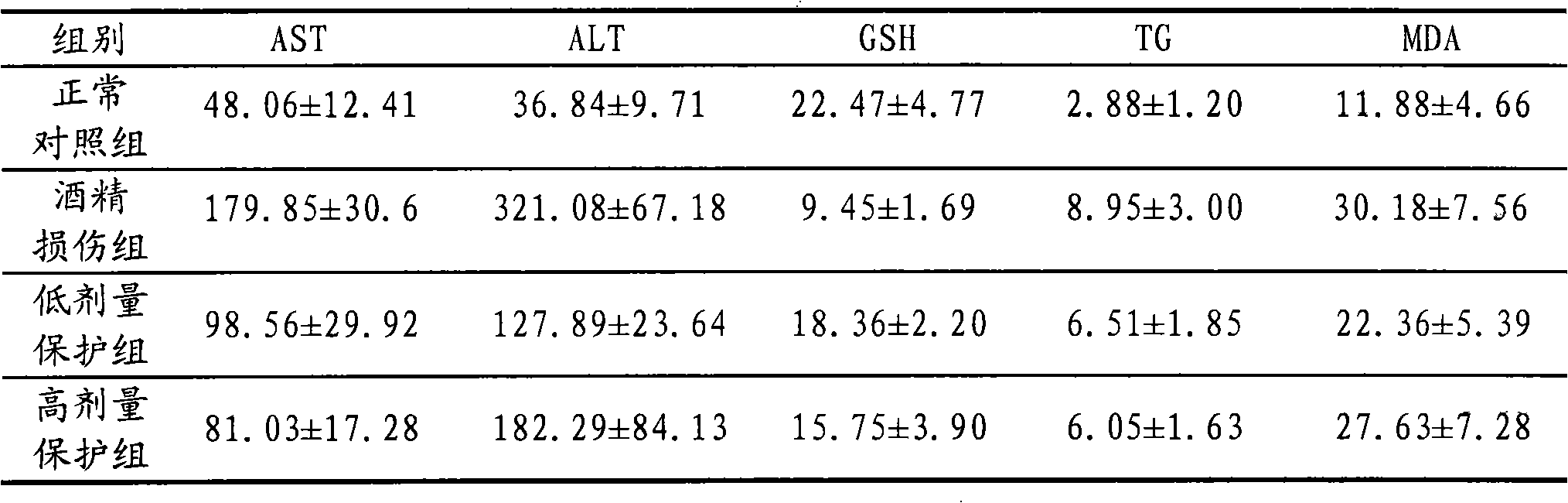
Health care product for relieving alcoholic liver
A health care product and product technology, applied in the field of health care products for alleviating alcoholic liver, can solve problems such as ineffective effects, and achieve the effects of enhancing the body's immunity, promoting gluconeogenesis, and improving taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 40g of L-arabinose, 15g of trehalose, 25g of fructooligosaccharide, 0.2g of selenium-enriched yeast, and 0.8g of magnesium stearate. After drying at 80°C, add 19g of mixed bacteria, mix evenly, and pack.
[0041] In the obtained product, L-arabinose accounts for 40% of the total mass of the product, trehalose accounts for 15% of the total mass of the product, mixed bacteria accounts for 19% of the total mass of the product, fructooligosaccharides accounts for 25% of the total mass of the product, selenium-enriched yeast Accounting for 0.2% of the total mass of the product, magnesium stearate accounts for 0.8% of the total mass of the product. Routine physical and chemical tests and microbial pathogen tests are carried out on the products, and the test results show that the products are qualified.
Embodiment 2
[0043] Weigh 30g of L-arabinose, 12g of trehalose, 30g of fructooligosaccharide, 5g of selenium-enriched yeast, and 8g of magnesium stearate. After drying, add 15g of mixed bacteria, mix evenly, and pack.
[0044] In the obtained product, L-arabinose accounts for 30% of the total mass of the product, trehalose accounts for 12% of the total mass of the product, mixed bacteria accounts for 15% of the total mass of the product, fructooligosaccharides accounts for 30% of the total mass of the product, selenium-enriched yeast It accounts for 5% of the total mass of the product, and magnesium stearate accounts for 8% of the total mass of the product. The product is subjected to routine physical and chemical inspection and microbial pathogenic bacteria inspection, and the inspection results show that the product is qualified.
Embodiment 3
[0046] Weigh 80g of L-arabinose, 1g of trehalose, 5g of fructooligosaccharide, 4g of selenium-enriched yeast, and 5g of magnesium stearate. After drying, add 5g of mixed bacteria, mix evenly, and pack.
[0047] in the obtained product. L-arabinose accounts for 80% of the total product mass, trehalose accounts for 1% of the total product mass, mixed bacteria accounts for 5% of the total product mass, fructooligosaccharides accounts for 5% of the total product mass, and selenium-enriched yeast accounts for the total product mass 4%, magnesium stearate 5% of the total product weight. Routine physical and chemical tests and microbial pathogen tests are carried out on the products, and the test results show that the products are qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com