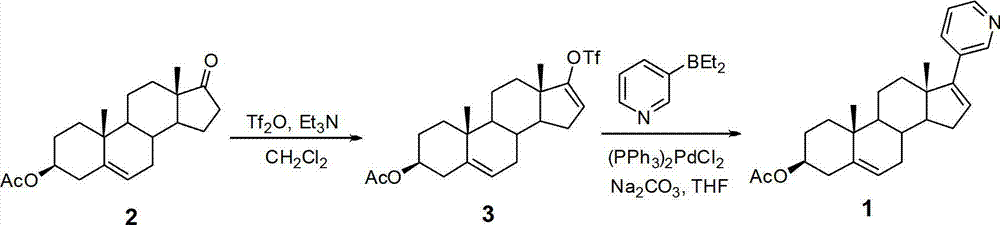
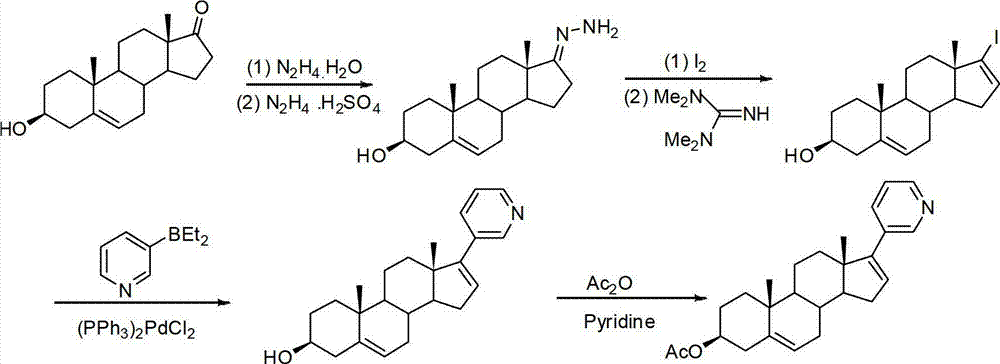
Method for preparing abiraterone acetate
A technology of alcohol acetate and catalyst, applied in the direction of steroids, organic chemistry, etc., can solve the problems of high price, high price and high cost of trifluoromethanesulfonic anhydride, achieve good application prospects, easy post-processing, and promote smooth progress Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
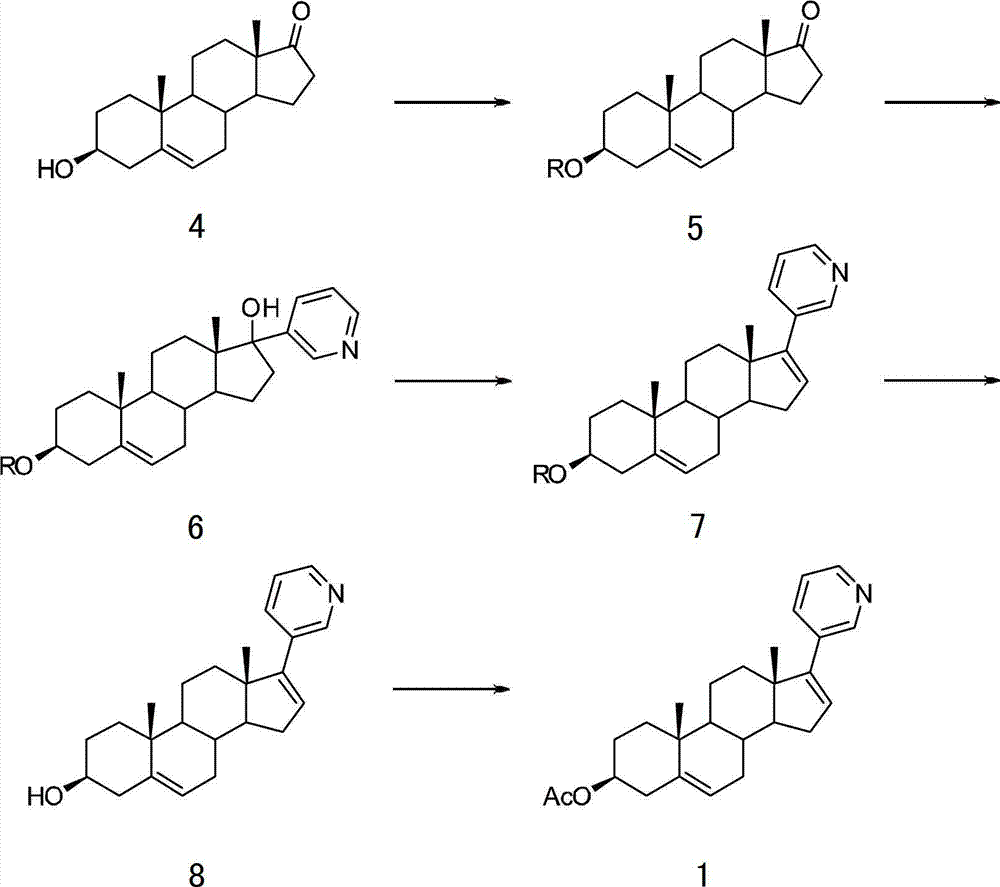
[0041] Embodiment 1: the preparation of compound shown in formula 5
[0042]
[0043] Add 3 g (10.4 mmol) of compound 3β-hydroxydhydrocorticosterone (Formula 4) into a 250 ml flask, and stir to dissolve with 75 mL of dichloromethane. 2.74 g (22.5 mmol) of 4-dimethylaminopyridine (DMAP), 3.3 mL (23.7 mmol) of triethylamine, and 4.5 g (29.8 mmol) of tert-butyldimethylsilyl chloride (TBDMSCl) were slowly added in portions. Stir at 25°C for 15 hours. Extract with 100ml of dichloromethane solution, wash the organic phase with 10% ammonium chloride solution and saturated brine, and dry over anhydrous sodium sulfate. Concentration under reduced pressure, followed by recrystallization to obtain about 4.1 g of compound 5, with a yield of about 97%.
[0044] 1 H NMR (300MHz, CDCl 3 )δ0.06(s, Me 2 Si), 0.88(s, Me(18)), 1.03(s, Me(19)), 0.89(s, t-Bu), 3.49(m, H-C(3)), 5.34(b r.d, J=5 ,H-C(6));
[0045] 13 C NMR (CDCl 3 ,100MHz)δ221.2,142.2,120.4,72.6,51.8,50.3,47.6,42.8,37.2,36....
Embodiment 2
[0047] Embodiment 2: the preparation of compound shown in formula 6
[0048]
[0049]
[0050] Add 37.6g (153.0mmol) of dry cerium trichloride to a 1L flask, add 300ml of pre-cooled anhydrous tetrahydrofuran at 0°C, slowly raise the temperature to 25°C and stir for 2 hours, then cool to 0°C for later use.
[0051] In another 1L flask, 4.4g (183.6mmol) of magnesium chips, 200ml of anhydrous tetrahydrofuran, and 14.8ml (153.0mmol) of 3-bromopyridine were added, and the mixture was refluxed at 80°C for 3 hours. After cooling to 0°C, it was added to the above pre-prepared cerium trichloride solution. After stirring at 0°C for 2 hours, slowly add 4.1 g (10.2 mmol) of tetrahydrofuran solution of 3β-(tert-butyldimethyl)silyloxydorcorticosterone (Formula 5) dropwise, and react at 0°C for 4 hours, Add water to quench. Concentrate under reduced pressure to remove tetrahydrofuran, extract with chloroform, wash the organic layer with saturated brine, and dry over anhydrous sodium ...
Embodiment 3
[0055] Embodiment 3: the preparation of compound shown in formula 7
[0056]
[0057] Add 4.5g of the compound (Formula 6) prepared above into a 1L flask, dissolve it in 100mL of anhydrous dichloromethane, slowly add 58.2ml (418.3mmol) of triethylamine, 63.8ml (793.6mmol) of pyridine, Methanesulfonyl chloride 4.6ml (59.1mmol). After reacting at 25°C for 3 hours, concentrate under reduced pressure to remove pyridine, add chloroform and water to extract, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized to obtain 3.3g of compound 7, two steps The total yield is about 70%.
[0058] 1 H NMR (300MHz, CDCl 3 )δ8.62(s,H-Py),8.46(d,H-Py),7.65(d,H-Py),7.21(t,H-Py),5.99(s,H-C(16)),5.34 (b r.d, H-C(6)), 3.50(m, H-C(3)), 1.04(s, Me(18)), 1.06(s, Me(19)), 0.89(s, t-Bu), δ0 .06(s, Me 2 Si);
[0059] 13 C NMR (CDCl 3 ,100MHz)δ151.6,147.9,147.8,141.8,133.6,132.9,129.2,122.9,120.8,72.5,57....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com