Preparation method of abiraterone acetate
A technology of abiraterone acetate and abiraterone, which is applied in the directions of steroids, chemical recovery, organic chemistry, etc., can solve the problems of cumbersome operation, inability to realize large-scale production, etc., achieves optimized reaction operating conditions, and is suitable for industrial scale-up The effect of production and reaction cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087]1. Preparation of crude abiraterone: In a 100L reaction kettle, add KOH (3.36kg, 60.0mol) and 32kg water to stir to dissolve, then add THF32L, 17-iodo-androster-5,16-diene-3β in sequence -Alcohol acetate (7.96kg, 20.0mol), bis(triphenylphosphine) palladium chloride [Pd(PPh 3 ) 2 Cl 2 ] (140g, 0.2mol), diethyl (3-pyridyl) borane (2.94kg, 20.0mol) was heated to reflux, and the reaction was stirred for 24 hours. The reaction solution was concentrated under reduced pressure to remove THF, and a large amount of solid was precipitated. Add 80L of water to the reaction kettle again, and continue to stir for 20 minutes. A large amount of light gray solid was obtained by centrifugation. The solid was rinsed with a large amount of water to pH 7-8, and the obtained solid was dried at 50-60° C. to obtain 7.23 kg of crude abiraterone.
[0088] 2. Preparation of Abiraterone Acetate:
[0089] 2.1 Purification of Abiraterone
[0090] Add the crude abiraterone obtained in step 1 in...
Embodiment 2
[0098] Add 50 g of the crude abiraterone acetate product prepared in step 2.2 of Example 1 into 500 ml of ethanol, heat and stir, and after it is completely dissolved, add 2.5 g of activated carbon, reflux for 0.5 h, and filter while it is hot. Add 500ml of purified water to the obtained filtrate, stir to precipitate crystals for 2 hours, and after the system reaches room temperature, cool to 0-5°C, and continue to stir to precipitate crystals for 12 hours. The white solid obtained by filtration was dried at 50-60° C., and dried to a constant weight to obtain 37.3 g of white crystals, with a mass yield of 74.5%.
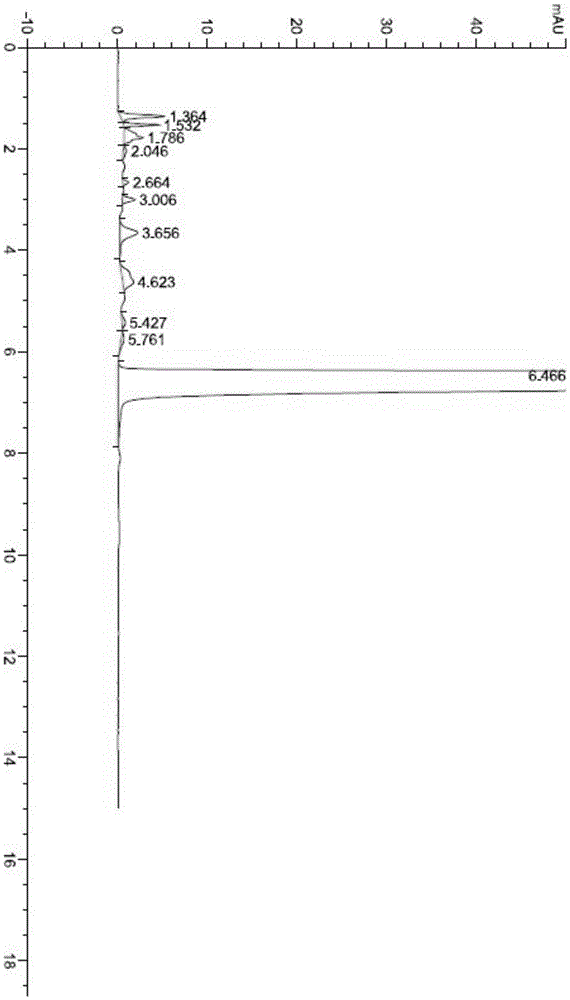
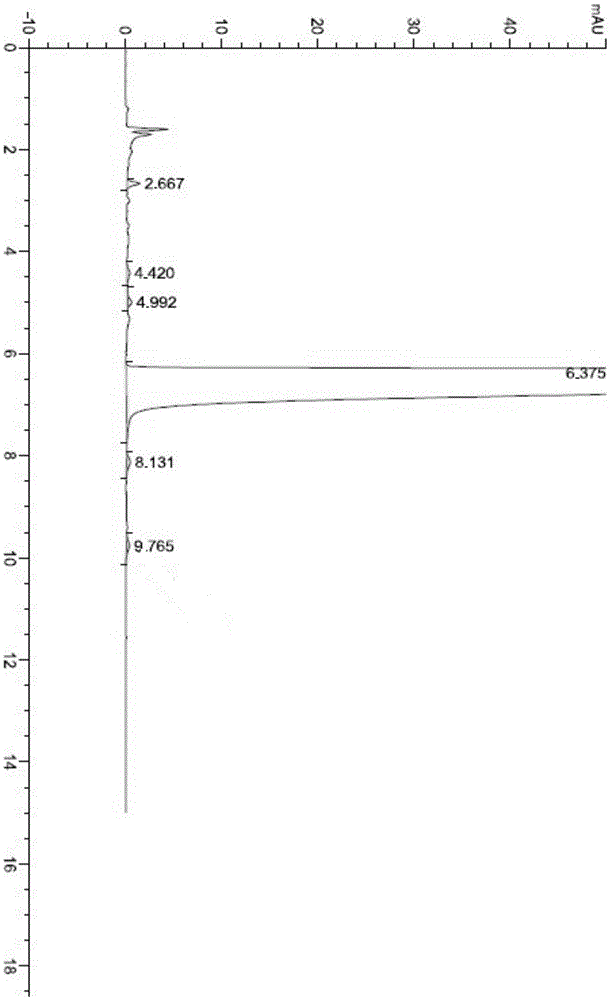
[0099] The HPLC test purity is 99.79%, the largest single impurity is 0.058%, the specific test chart is shown in the attached picture image 3 .
Embodiment 3
[0101] Add 50 g of the crude abiraterone acetate product prepared in step 2.2 of Example 1 into 400 ml of methanol, heat and stir, and after it is completely dissolved, add 2.5 g of activated carbon to reflux for 0.5 h, and filter while it is hot. Add 400ml of purified water to the obtained filtrate, stir to precipitate crystals for 2 hours, and after the system reaches room temperature, cool to 0-5°C, and continue to stir to precipitate crystals for 12 hours. The white solid was filtered and dried at 50-60° C., and dried to a constant weight to obtain 31.6 g of white crystals, with a mass yield of 63.2%.
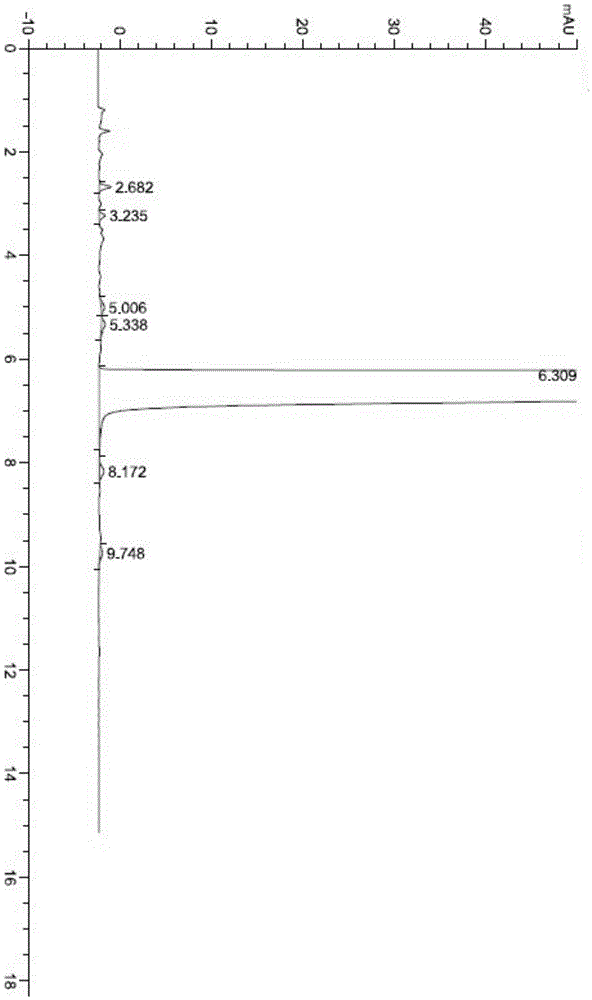
[0102] The HPLC test purity is 99.89%, the largest single impurity is 0.03%, and the specific test chart is shown in the attached picture Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com