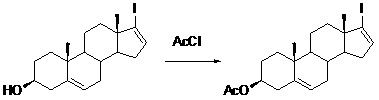Preparation method of abiraterone acetate
A technology of abiraterone acetate and abiraterone, which is applied in the field of medicine to achieve the effect of shortening the reaction steps and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 : Preparation of (3β)-17-iodo-androst-5,16-dien-3-ol acetate (compound II).
[0025]
[0026] I II
[0027] In a 250ml three-necked flask, add 39.8 g (0.1 mmol) of compound I, 300 ml of acetonitrile, and 20 g of triethylamine, and add 9.4 g (0.12 mmol) of acetyl chloride dropwise into the reaction system at room temperature. After dropping, heat up to 70°C and react for 4 hours. TLC spotting test shows that the reaction is complete. Cool to room temperature, distill and remove the solvent to obtain a residue. Add 50ml of water, extract with ethyl acetate (20ml×3), anhydrous sodium sulfate After drying, the desiccant was filtered off, and the filtrate was distilled under reduced pressure to obtain 38.7 g of white solid, yield: 97.9%.
[0028] MS(+1):441.
[0029] 1 HNMR: δ (ppm, CDCl 3 ), 6.41-6.42 (t, 1H), 5.37-5.39 (t, H), 3.41-3.43 (q, 2H), 2.87-2.89 (m, 1H), 2.21-2.23 (d, 1H), 2.124-2.13 (d, 1H), 2.01 (s, 3H), 1.96-1.97 (d...
Embodiment 2
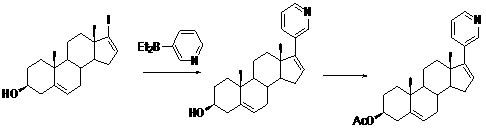
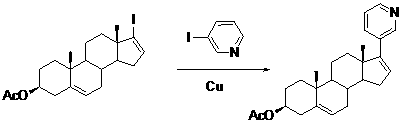
[0031] Example 2 : Preparation of Abiraterone Acetate (Compound TM)
[0032]
[0033] In a 250ml three-necked flask with reflux condensation, add 22.0 grams of (3β)-17-iodo-androst-5,16-dien-3-ol acetate (compound II, 0.05mol), 15.3g 3- Iodopyridine (compound III, 0.075 mol). Stir, add copper powder 15.8g (0.25mol), quickly heat up to 210°C, react for 4 hours, TLC detects that the reaction is complete, cool, add ethyl acetate, 100 ml each time, elute 3 times, combine the organic phase, 50mL saturated salt Wash once with water, separate the organic phase, dry over anhydrous sodium sulfate for 3 h, filter off the desiccant, and concentrate the organic phase to obtain an off-white solid. The obtained off-white solid was recrystallized once from ethyl acetate-petroleum ether (2:1, 300 mL), and the white solid was dried in vacuum to obtain 11.7 g. The obtained 11.7 g white solid was recrystallized from ethanol (120 mL), and the obtained white solid was vacuum-dried to obtain...
Embodiment 3
[0037] Example 3 : (Preparation of (3β)-17-iodo-androst-5,16-dien-3-ol acetate (compound II).
[0038]
[0039] I II
[0040] In a 250ml three-neck flask, add 39.8 g (0.1 mmol) of compound I, 300 ml of dichloromethane, and 18 g of sodium bicarbonate, and add 9.4 g (0.12 mmol) of acetyl chloride dropwise to the reaction system at room temperature. After dropping, heat up to 75°C to react for 4 hours, TLC spotting test shows that the reaction is complete, cool to room temperature, filter off the inorganic salt, distill the organic solvent, remove the solvent, and obtain a residue, add 50ml of water, extract with ethyl acetate (20ml× 3), dried over anhydrous sodium sulfate, filtered off the desiccant, and distilled the filtrate under reduced pressure to obtain 39.2 g of white solid, yield: 88.9%.
[0041] NMR data are the same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com