Abiraterone acetate crystal form and preparation method thereof
A technology of abiraterone acetate and crystal form, applied to a new crystal form of the prostate cancer treatment drug abiraterone acetate and its preparation, the pharmaceutical composition of the new crystal form, the application field in the treatment of prostate cancer, can solve the problem of Unable to inhibit androgen and other problems, to achieve good stability and activity, good preparation adaptability, suitable for long-term storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Preparation of Abiraterone Acetate Form E
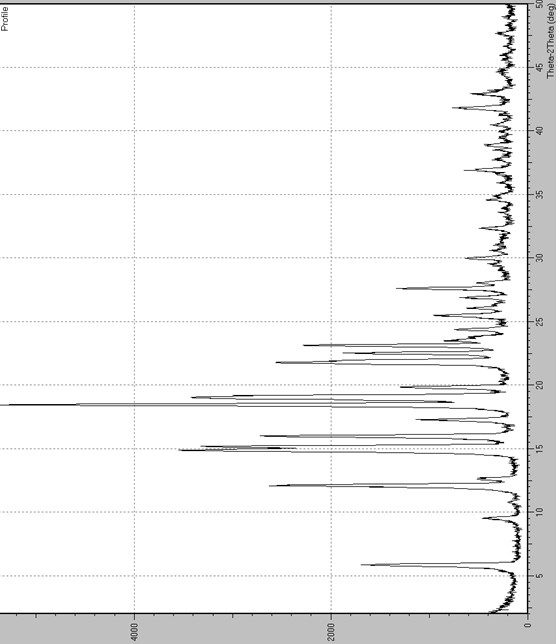
[0070] In a 100ml reaction bottle, add 10g of abiraterone acetate and 50ml of acetonitrile, heat up to reflux to dissolve completely, cool to below 10°C to crystallize at a stirring speed of 140~150 rpm, filter, and depressurize the obtained solid Dry to obtain the crystal form E of abiraterone acetate. The measured X-ray powder diffraction pattern is shown in figure 1 , the measured values are as follows (take peaks whose relative intensity is greater than or equal to 7%),
[0071] d value 2θ value Relative Strength(%) 15.12 5.8° 31 9.28 9.5° 7 7.31 12.1° 48 7.01 12.6° 7 5.95 14.9° 66 5.84 15.2° 62 5.54 16.0° 50 5.13 17.3° 17 4.81 18.4° 100 4.66 19.0° 65 4.48 19.8° 21 4.07 21.8° 46 3.95 22.5° 30 3.85 23.1° 40 3.78 23.5° 10 3.65 24.4° 10 3.50 25.5° 14 3.42 26.0° 7 3.31 26.9° 8 3.23 27.6° 23 ...
Embodiment 2
[0073] Preparation of Abiraterone Acetate Form E
[0074] In a 500ml reaction bottle, add 5g of abiraterone acetate and 80ml of methanol, dissolve the product completely at 30~35°C, add 160ml of water at a stirring speed of 50~60 rpm, crystallize, filter, and obtain a solid Dry under reduced pressure to obtain the crystal form E of abiraterone acetate.
[0075]
Embodiment 3
[0077] Preparation of Abiraterone Acetate Form E
[0078] In a 100ml reaction bottle, add 10g of abiraterone acetate and 50ml of ethanol, heat up to reflux to dissolve the product completely, cool to below 0°C to crystallize at a stirring speed of 190~250 rpm, and filter to obtain abiraterone acetate Dragonglass Form E.
[0079]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com