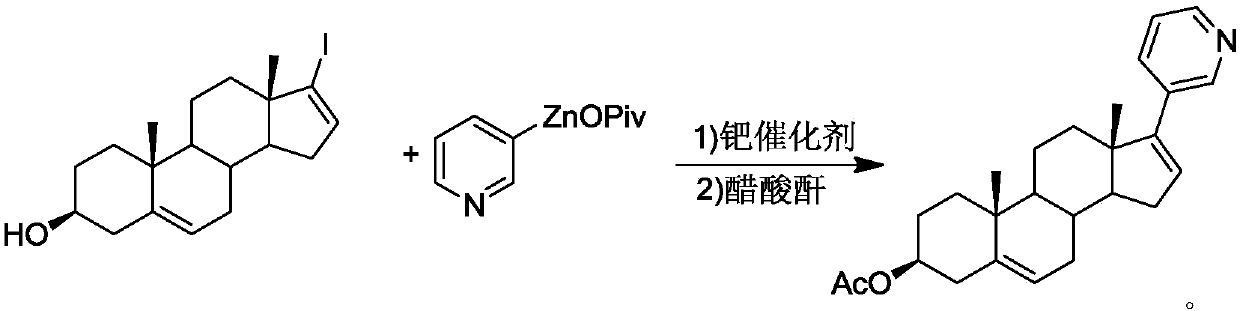
Abiraterone acetate preparation method
A technology of abiraterone acetate and palladium acetate, applied in directions such as steroids and organic chemistry, can solve problems such as limiting industrial application, and achieve the effects of large application value, simple production method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Add 63.2g (0.40mol, 1.0equiv) of raw material 3-bromopyridine into a 1000mL three-neck flask equipped with magnetic stirring and reflux condenser, add 500mL of dry tetrahydrofuran as a solvent, replace with nitrogen and cool to 0°C, slowly drop Add 352mL of isopropylmagnesium bromide lithium chloride solution (1.25M, 0.44mol, 1.1equiv) and drop it over one hour. After the dropwise addition was completed, the temperature was raised to 25° C. for 3 hours, and 123 g (0.46 mol, 1.15 equiv) of solid zinc pivalate was added. After the addition was completed, the reaction was continued for 30 minutes, concentrated under reduced pressure to dryness, and vacuum-dried for two hours ( Be careful not to have air entering the reaction flask), to obtain solid 3-pyridine zinc pivalate and salt mixture 279g (0.30 ~ 0.34mol, conversion rate 75% ~ 85%), the solid is directly used in the next step without separation , all feeds are calculated based on the content of 279 grams ...
Embodiment 2
[0031]
[0032]Add 39.8 g (0.10 mol, 1.0 equiv) of the reaction compound 17-iodandrost-5,16-diene-3β-hydroxyl, 3-pyridine neopentyl Zinc acid 93.0g (0.10mol, 1.0equiv), [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridine)palladium dichloride (PEPPSI-IPr) 0.68g (0.001mol, 0.01equiv), add 500mL tetrahydrofuran as a solvent, replace with nitrogen and heat to 50°C for 3 hours. After the reaction was completed, the solvent was removed by distillation under reduced pressure (distillation temperature was 40°C, vacuum degree was -0.08MPa), and a solution of 500 mL of dichloromethane and 11.1 g (0.11 mol, 1.1 equiv) of triethylamine was added, and 4-dimethylamino Pyridine 0.62g (0.005mol, 0.05equiv) was used as a catalyst, the temperature was lowered to 0°C, and 15.3g (0.15mol, 1.5equiv) of acetic anhydride was slowly added dropwise. After the addition was completed, the reaction was continued for three hours after the temperature was raised to 25°C, and filtered. W...
Embodiment 3
[0034]
[0035] Add 39.8 g (0.10 mol, 1.0 equiv) of the reaction compound 17-iodandrost-5,16-diene-3β-hydroxyl, 3-pyridine neopentyl Zinc acid 111.6g (0.12mol, 1.2equiv), dichloroditriphenylphosphine palladium 3.5g (0.005mol, 0.05equiv), add ethanol 500mL as a solvent, replace with nitrogen and heat to 70°C for 3 hours. After the reaction was completed, the solvent was removed by distillation under reduced pressure (the distillation temperature was 50°C, and the vacuum degree was -0.08MPa), and a solution of 500 mL of dichloromethane and 11.1 g (0.11 mol, 1.1 equiv) of triethylamine was added, and 4-dimethylamino Pyridine 0.62g (0.005mol, 0.05equiv) was used as a catalyst, the temperature was lowered to 0°C, and 15.3g (0.15mol, 1.5equiv) of acetic anhydride was slowly added dropwise. After the addition was completed, the reaction was continued for three hours after the temperature was raised to 25°C, and filtered. Wash the organic layer with 500mL of water and 500mL of 1N s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com