Solid dispersion and preparation method thereof
A technology of solid dispersion and carrier material, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
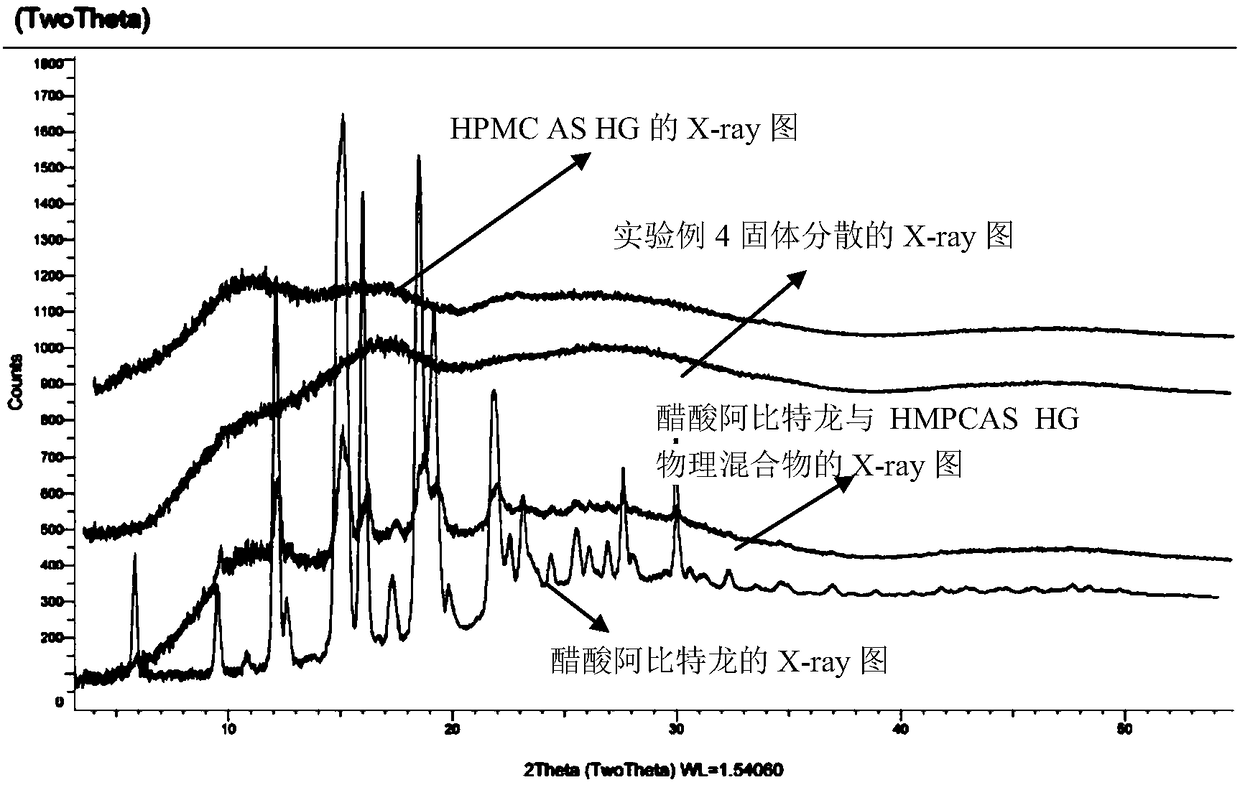
Image
Examples
Embodiment 1
[0045] Embodiment 1: preparation of solid dispersion
[0046] Compound A and different types of carrier materials were used to prepare solid dispersions. The specific formula is shown in Table 2:
[0047] Table 2
[0048]
[0049] Preparation:
[0050] 1) Pass the abiraterone acetate and the carrier material through a 40-80 mesh screen respectively, weigh the abiraterone acetate and the carrier material in a ratio of 1:3 by weight percentage, and fully mix them uniformly;
[0051] 2) Preset the extrusion temperature of each heating zone of twin-screw hot melt extruder 1-8 to 120°C-145°C, when the preset temperature is reached, start the screw to slowly add the mixture obtained in step 1) from the feeding hopper to the extruder In the machine, hot melt extrusion is carried out at a speed of 150-300RPM;
[0052] 3) The solid extrudate obtained in step 2) is pulverized and passed through a 60-100 mesh sieve to obtain a uniform solid dispersion of particles.
Embodiment 2
[0054] Compound A and different types of carrier materials were used to prepare solid dispersions. The specific formula is shown in Table 3:
[0055] table 3
[0056]
[0057] Preparation:
[0058] Dissolve abiraterone acetate and carrier material in dichloromethane / methanol mixed solvent, according to the ratio in Table 3, fully stir evenly, prepare the solution, use spray drying equipment to carry out spray drying, and the spray-dried product is dried in a vacuum oven. The obtained product is an amorphous solid dispersion of abiraterone acetate.
Embodiment 3
[0060] Compound A and different types of carrier materials were used to prepare solid dispersions. The specific formula is shown in Table 4:
[0061] Table 4
[0062]
[0063] Preparation:
[0064] The abiraterone acetate and the carrier material are dissolved together in the dichloromethane / methanol mixed solvent, and the organic solvent is evaporated to allow the drug and the carrier material to be precipitated at the same time, that is, a coprecipitate formed by mixing the abiraterone acetate and the carrier material is obtained. After drying, the solid dispersion of abiraterone acetate was obtained.
[0065] Appearance and Yield
[0066] The solid dispersions of Examples 1-3 and Comparative Examples 1-6 were compared for appearance and recovery data.
[0067] table 5
[0068] Element
Exterior
Recovery rate(%)
Experimental example 1
dry dispersion
82
Experimental example 2
dry dispersion
80
Experimental example 3
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com