Nitrendipine sustained-release preparation and preparation method thereof
A technology of nitrendipine and nitren, applied in the field of sustained-release pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
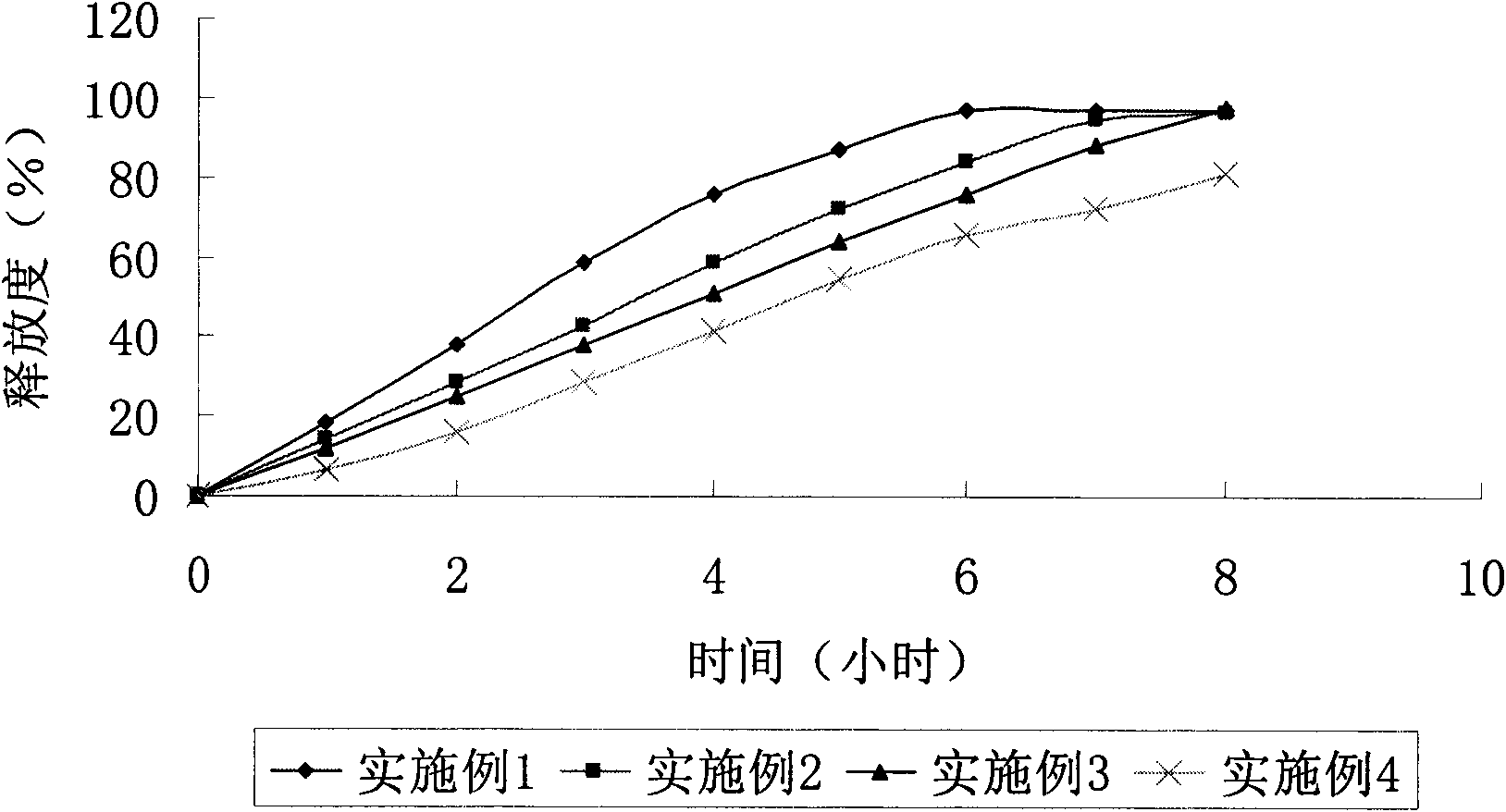
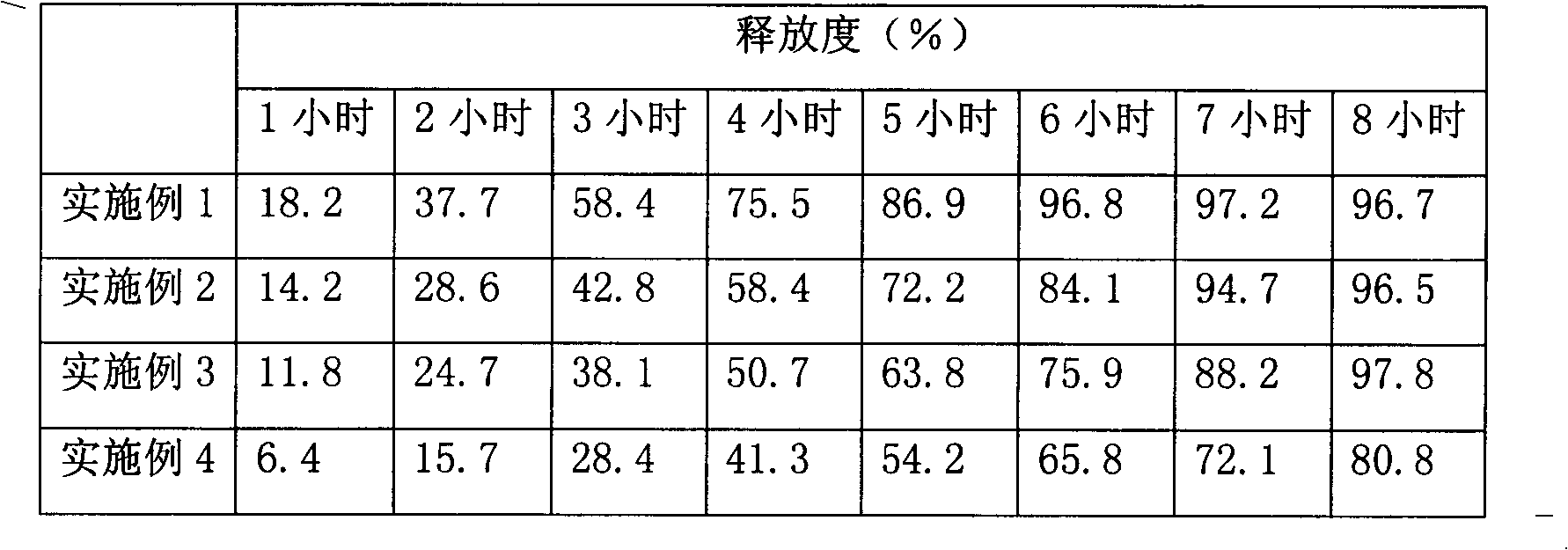
Embodiment 1
1000 pieces
[0036] Preparation process
[0037] 1 Preparation of adhesive: Dissolve the povidone of the prescribed amount in ethanol to make a 5% (w / w) adhesive solution for subsequent use;
[0038] 2 Granulation Mix nitrendipine, lactose, and ethyl cellulose evenly, add a binder for wet granulation, take out the granules and use a 20-mesh sieve for wet granulation. After drying, pass through a 20-mesh sieve for granulation and set aside.
[0039] 3. Tablet preparation. After mixing the prepared granules with the prescribed amount of magnesium stearate evenly, test the content to determine the tablet weight, and press into tablets to obtain.
Embodiment 2
[0042] Preparation process
[0043] 1 Preparation process of plain tablets
[0044] 1.1 Preparation of dispersion Take the prescribed amount of nitrendipine and copovidone and dissolve them in ethanol to make a 5% (w / w) copovidone ethanol dispersion for subsequent use;
[0045] 1.2 Granulation Put hypromellose, starch, sucrose, and micropowder silica gel into a mixing granulator, mix them, and add copovidone ethanol dispersion for wet granulation after uniformity, take out the granules and use a 12-mesh sieve for wet sizing Granules, the granules are ventilated and dried below 50°C. The dry granules are tested for water content, and after the test is qualified (the water content of the granules is 2.0-4.0%), the granules are sized with a 12-mesh sieve and set aside.
[0046]1.3 Tablet compression: After mixing the prepared granules with stearic acid in the prescribed amount evenly, test the content to determine the tablet weight, and then press into tablets to obtain ...
Embodiment 3
Appropriate amount
[0055] Makes 1000 pieces
[0056] Preparation process
[0057] 1 Preparation process of plain tablets
[0058] 1.1 Preparation of adhesive: Dissolve the povidone of the prescribed amount in ethanol to make a 6% (w / w) adhesive for subsequent use;
[0059] 1.2 Granulation Put nitrendipine, alginic acid, lactose, and mannitol together into a mixing granulator, mix them, and add a binder to carry out wet granulation after uniformity. Take out the granules and use a 16-mesh sieve for wet granulation. After drying, pass through a 16-mesh sieve for granulation and set aside.
[0060] 1.3 Compression: After mixing the prepared granules with the prescribed amount of magnesium stearate evenly, test the content to determine the tablet weight, and then compress the tablet.
[0061] 2 coating process
[0062] Take cellulose acetate, add 800ml of acetone, stir to dissolve; take another polyethylene glycol, put it in a 100ml measuring bottle, add water to di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com