Lurasidone solid dispersion and preparation method thereof
A technology of solid dispersion and lurasidone, which is applied in the field of medicine, can solve the problems of delaying the patient's condition, affecting the curative effect, and decreasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
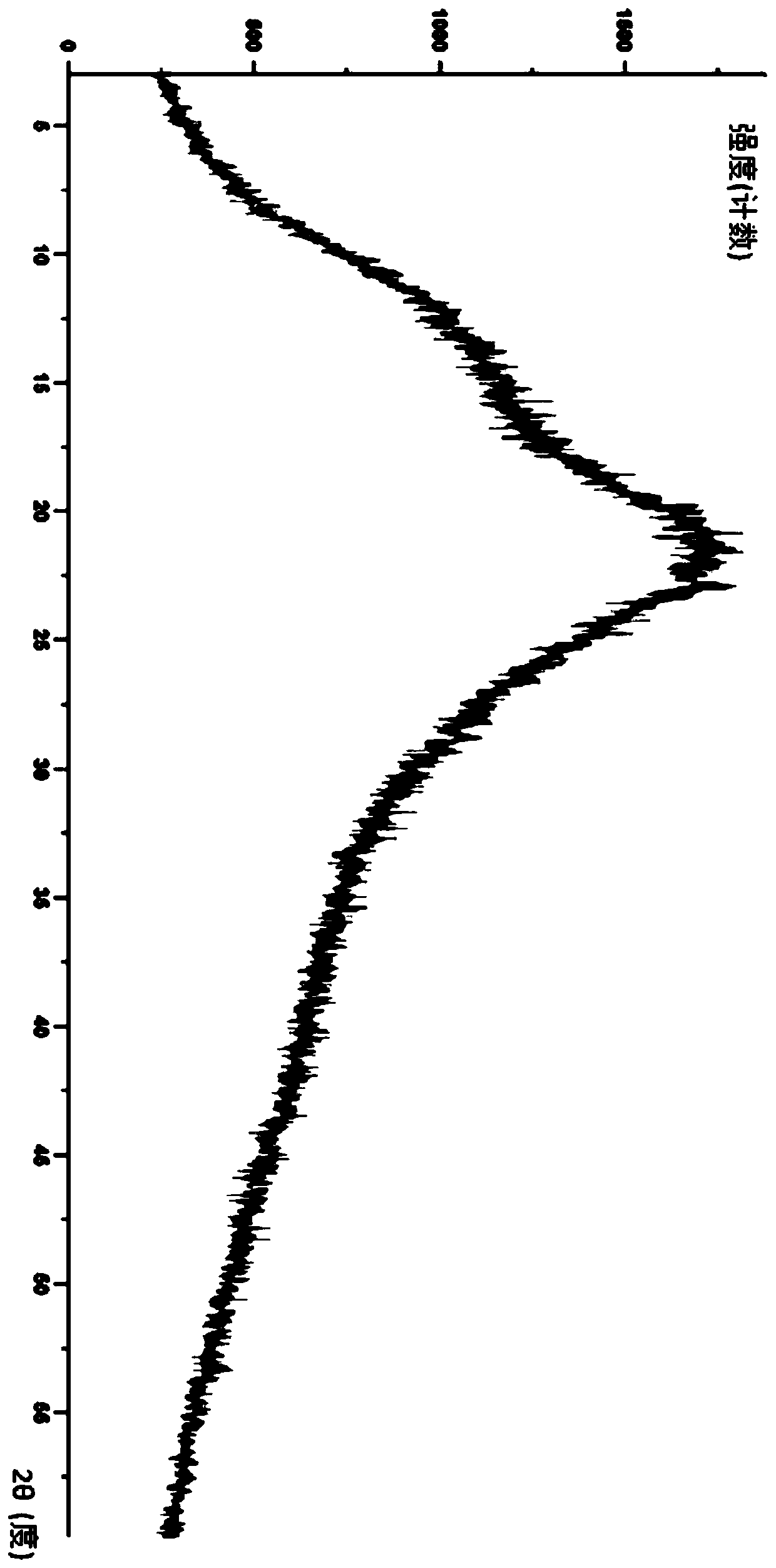
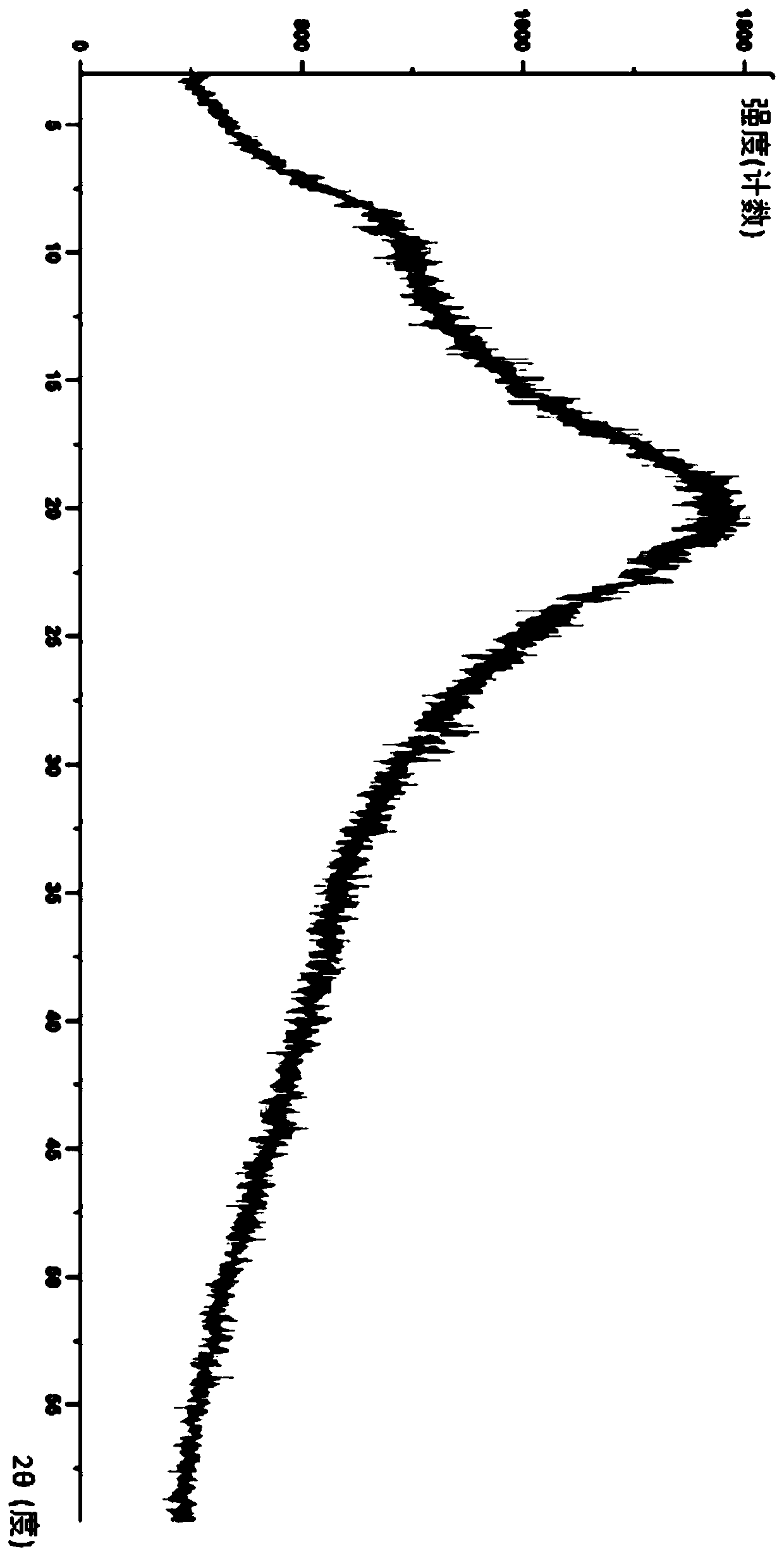
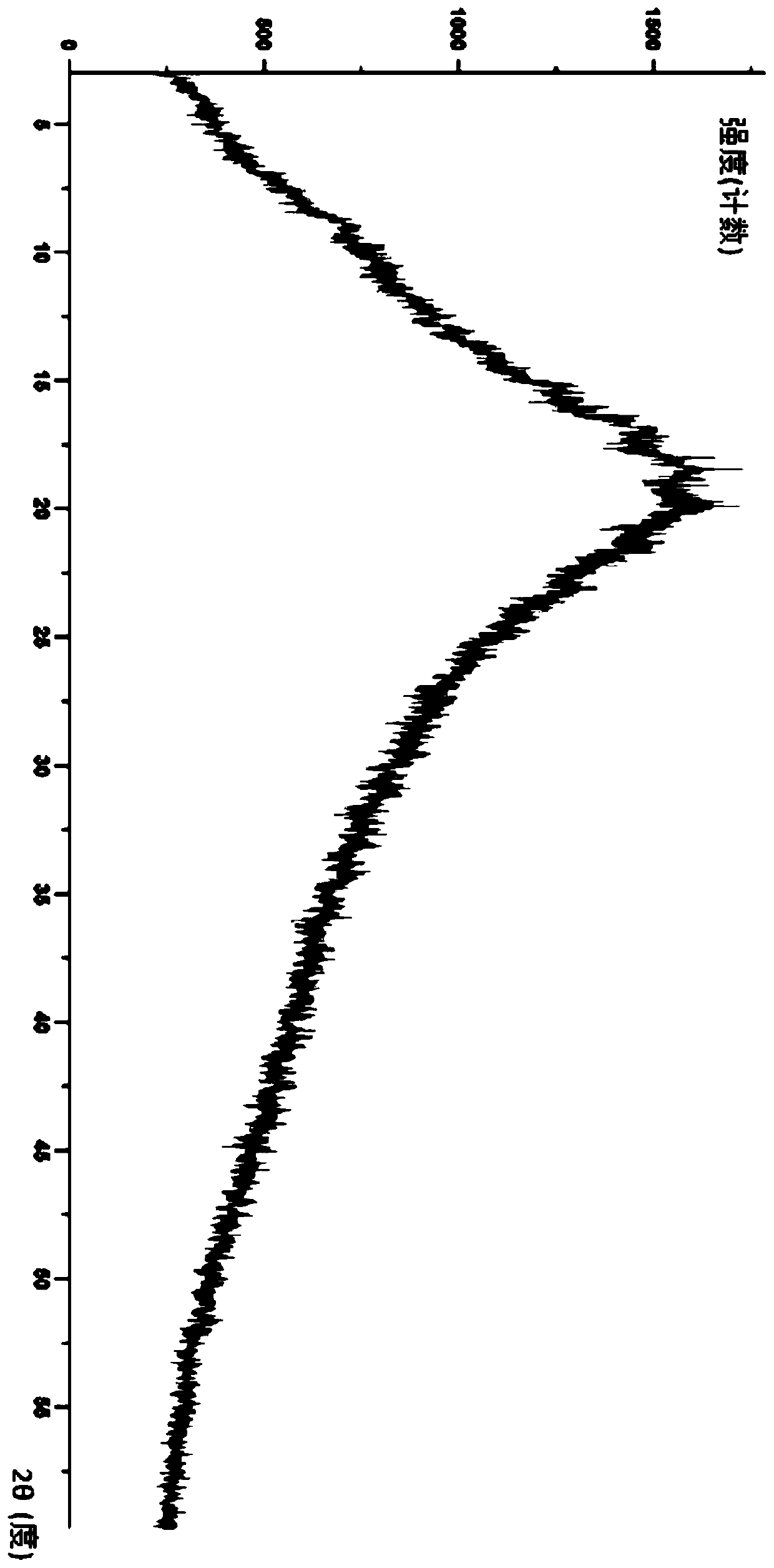
Image
Examples
Embodiment 1
[0100] The specific prescription is shown in Table 7.
[0101] Table 7
[0102]
[0103] Weigh the components contained in the hot-melt process according to the prescription shown in Table 7, mix them thoroughly and add them to the powder feeder of the hot-melt extruder. ℃, 165℃, 165℃, 165℃, 160℃, the screw speed is set to 30rpm, hot-melt extrusion, the extruded product is cooled and crushed, the crushed material passes through a 60-mesh sieve, and the additive microcrystal is added according to the prescription The cellulose is mixed, and the resulting mixture is filled into capsules.
Embodiment 2
[0105] According to the prescription and preparation process of Example 1, the obtained granules are finally poured into the shell of enteric-coated capsules.
Embodiment 3
[0107] The specific prescription is shown in Table 8.
[0108] Table 8
[0109]
[0110]
[0111] Weigh the components contained in the hot-melt process according to the prescription shown in Table 8, mix them thoroughly and add them to the powder feeder of the hot-melt extruder. ℃, 170℃, 170℃, 170℃, 165℃, the screw speed is set to 30rpm, hot-melt extrusion, the extrudate is cooled and crushed, the crushed material passes through a 60-mesh screen, and the additive microcrystal is added according to the prescription Cellulose, mixed, filled with capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com