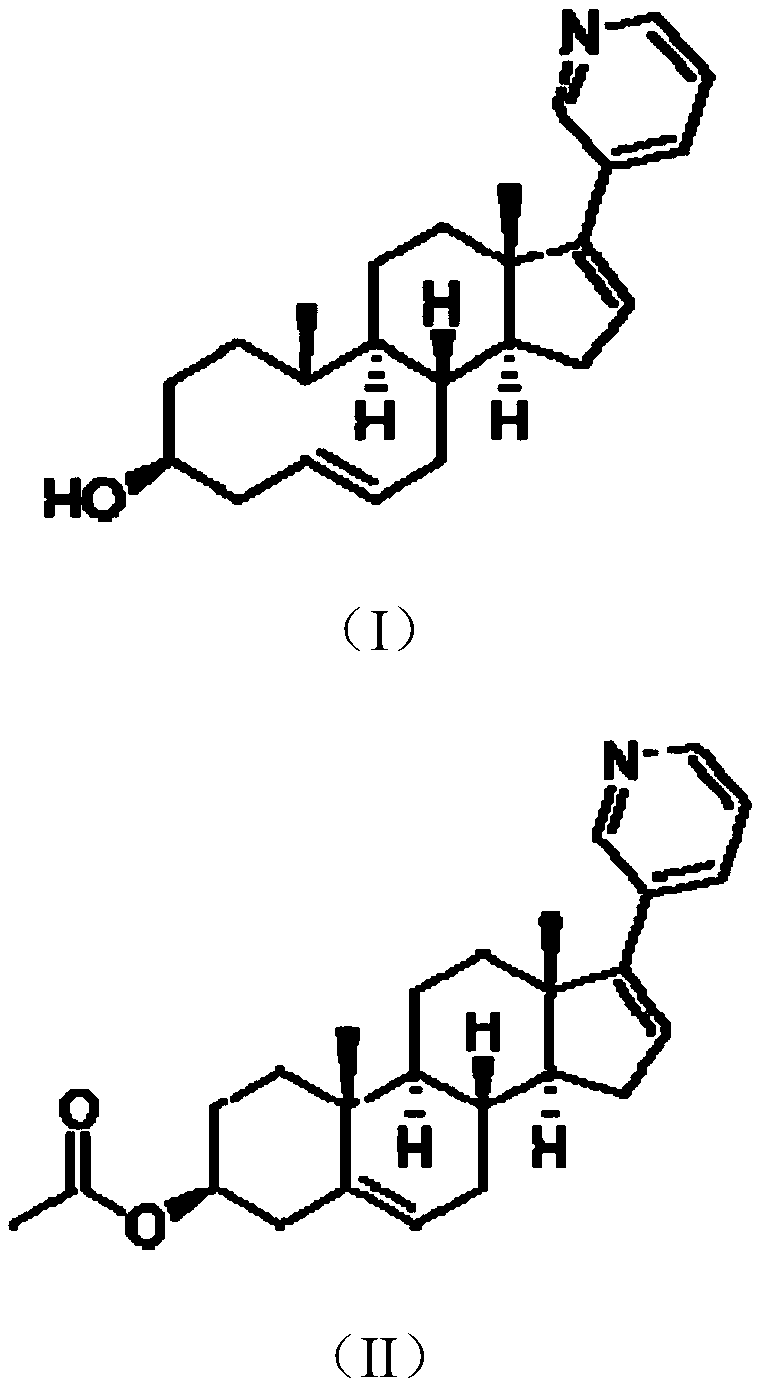
Abiraterone oral emulsion and preparation method thereof
A technology of abiraterone and abiraterone acetate, which is applied in the direction of pharmaceutical formulations, emulsion delivery, medical preparations of non-active ingredients, etc., can solve the problems of low bioavailability, achieve improved dissolution, improved bioavailability, The effect of emulsion stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Excipient investigation
[0040] Table 1: Solubility investigation of excipients
[0041]
[0042]
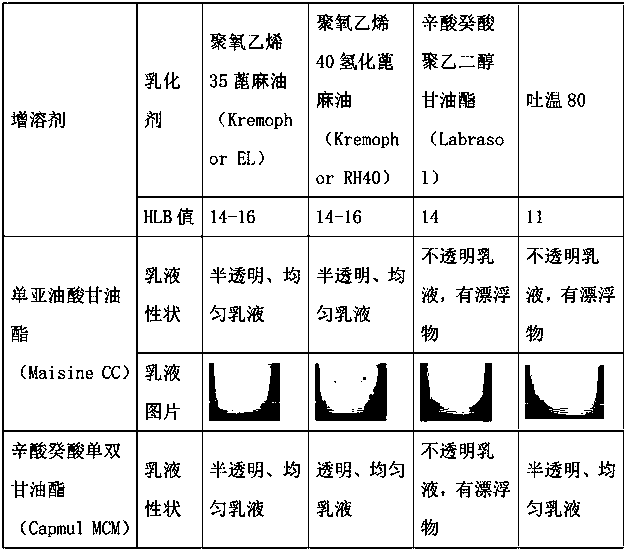
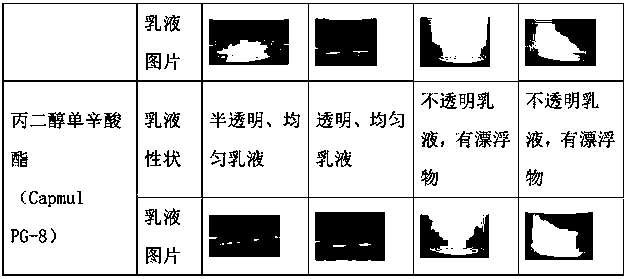
[0043] Table 2: Investigation of emulsifying properties of excipients
[0044]
[0045]
[0046] Table 3: Investigation on the Stability of Excipients
[0047]
[0048]
[0049] The results showed that: (1) Abiraterone acetate was easily degraded after being dissolved in ester excipients; (2) Adding antioxidants could effectively improve the stability of abiraterone acetate in the emulsion.
Embodiment 2
[0050] Embodiment 2: the preparation of abiraterone oral emulsion
[0051] A preparation method of abiraterone emulsion comprises the following steps:
[0052] (1) After heating the solubilizer and emulsifier to 60°C, stir evenly to obtain solution 1;
[0053] (2) Add antioxidant to solution 1, stir and dissolve to obtain solution 2;
[0054] (3) Add the active ingredient into solution 2, stir to dissolve, and obtain the emulsion.
Embodiment 3
[0055] Example 3: Emulsion Evaluation
[0056] The emulsion was prepared according to the method described in Example 2 according to the proportion of the components. Weigh 1g of the emulsion, add it to 9ml of purified water, stir to dissolve, and observe the properties of the emulsion.
[0057] (1) Simulated gastric digestion evaluation:
[0058] According to the literature Koziolek, M., et al. (2019). "The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group." European Journal of Pharmaceutical Sciences 134:31-59. The amount of fluid in the stomach is affected by the state of eating The impact is relatively large. In the fasting state, the amount of fluid in the stomach is about 35ml, and the pH is about 1. Firstly, 5g of emulsion was diluted into 50ml of emulsion, then mixed with 35ml of artificial gastric juice (Chinese Pharmacopoeia, containing pepsin), and simulated gastric digestion at 37°C for 1 hour. After digestion, the sample is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com