Stable abiraterone oral solid medicinal composition and preparation method thereof
A technology of abiraterone and abiraterone acetate, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
[0034] For the prescription of citrate, the detailed prescription is shown in Table 1:
[0035] Table 1 The prescription form of Examples 1-7 (comprising citric acid or its pharmaceutically acceptable salt)
[0036] prescription Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Example 7 Abiraterone acetate 125mg 250mg 250mg 250mg 250mg 250mg 500mg Sodium citrate (as anhydrous sodium citrate) 1.25mg —— 10mg 12.5mg 16.7mg —— 100mg Potassium citrate (calculated as anhydrous potassium citrate) —— 5mg —— —— —— 25mg —— lactose —— 200mg 200mg 200mg 200mg 200mg —— microcrystalline cellulose —— 160mg 160mg 160mg 160mg 160mg —— Mannitol 250mg —— —— —— —— —— 125mg starch —— —— —— —— —— —— 125mg pregelatinized starch 250mg —— —— —— —— —— —— Croscarmellose Sodium —— 40mg 40mg 40mg 40mg 40mg —— Cros...
Embodiment and
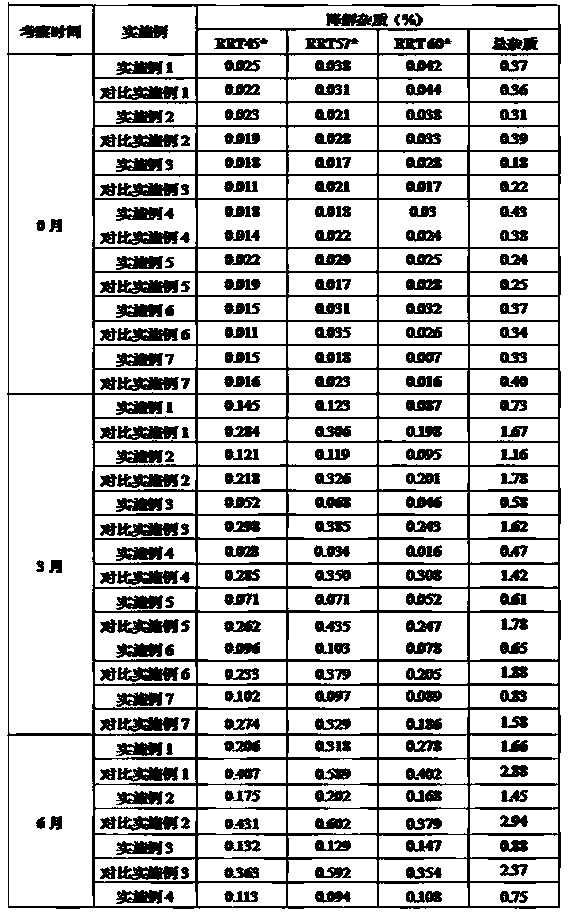
[0067] Embodiment and comparative examples 1-7 place 2 months under the condition of 60 ± 2 ℃ of degraded impurity comparison
[0068] The samples of Examples and Comparative Examples 1-7 placed at 60±2°C for 2 months were measured according to the above-mentioned method for measuring degraded impurities, and the results are shown in Table 3.
[0069] Table 3 Degradation Impurity Determination Results of Examples and Comparative Examples 1-7 placed at 60±2°C for 2 months
[0070]
[0071] Note: * indicates three degradation impurities whose relative retention time with abiraterone acetate is 0.45, 0.57, 0.60.
[0072] The results of the comparison of the degraded impurities in the above examples and comparative examples 1 to 7 show that the three main degraded impurities RRT45, RRT57, and RRT60 was significantly reduced, indicating that citrate can significantly improve the stability of each formulation (composition).
[0073]
Embodiment
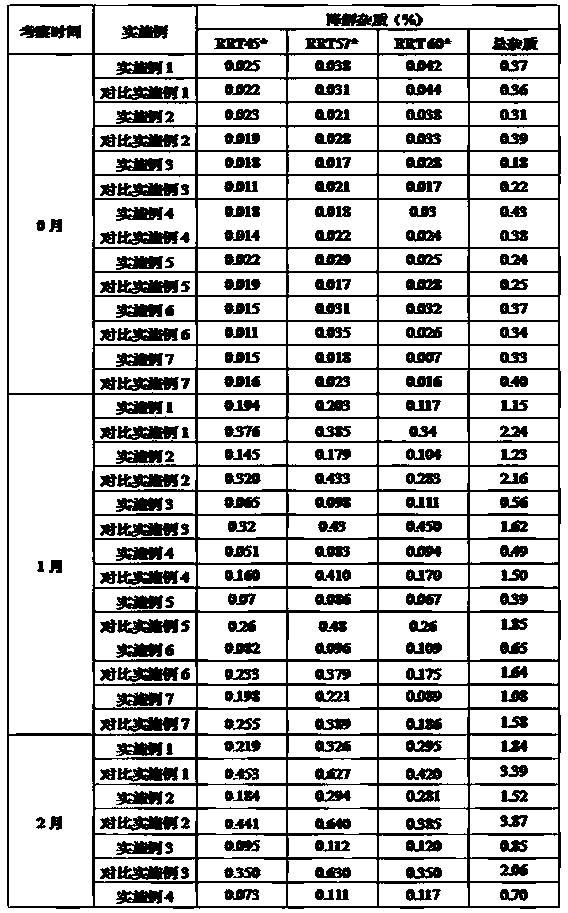
[0074] Embodiments and comparative examples 1-7 place 6 months under the condition of 40 ± 2 ℃, RH75% ± 5% degradation impurity comparison
[0075] The samples of Examples and Comparative Examples 1 to 7 placed for 2 months under the conditions of 40±2°C and RH75%±5% were measured according to the above-mentioned determination method of degraded impurities, and the measurement results are shown in Table 4.
[0076] Table 4 Degradation Impurity Determination Results of Examples and Comparative Examples 1-7 placed at 40±2°C for 6 months
[0077]
[0078] Note: * indicates three degradation impurities whose relative retention time with abiraterone acetate is 0.45, 0.57, 0.60.
[0079] The comparative measurement results of the degradation impurities of the above examples and comparative examples 1 to 7 show that at 40 ± 2°C 、 Placed for 6 months under the condition of RH75%±5%, the three main degradation impurities RRT45, RRT57 and RRT60 were significantly reduced after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com