Abiraterone acetate polymorphic substance and pharmaceutical composition
A technology for abiraterone acetate and polymorphs, which can be used in drug combinations, steroids, pharmaceutical formulations and other directions, and can solve the problems of crystal form destruction threats, poor crystal fluidity, and unfavorable crystal form stable storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The preparation of embodiment 1 polymorph I
[0099] plan 1,
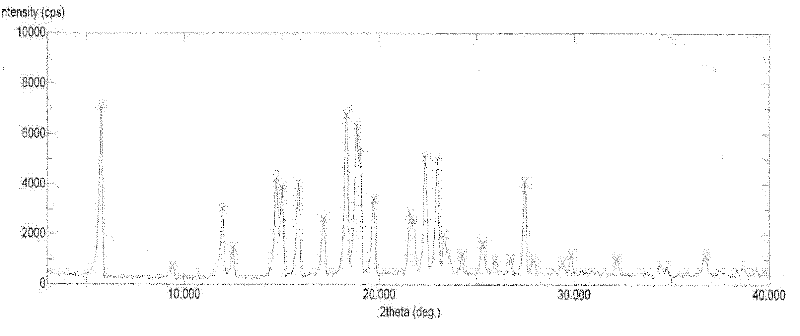
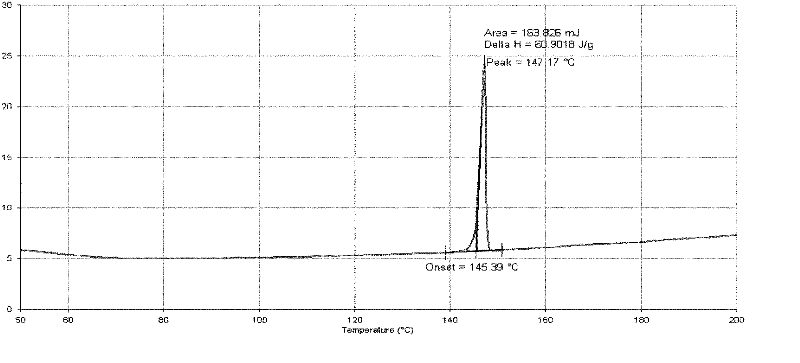
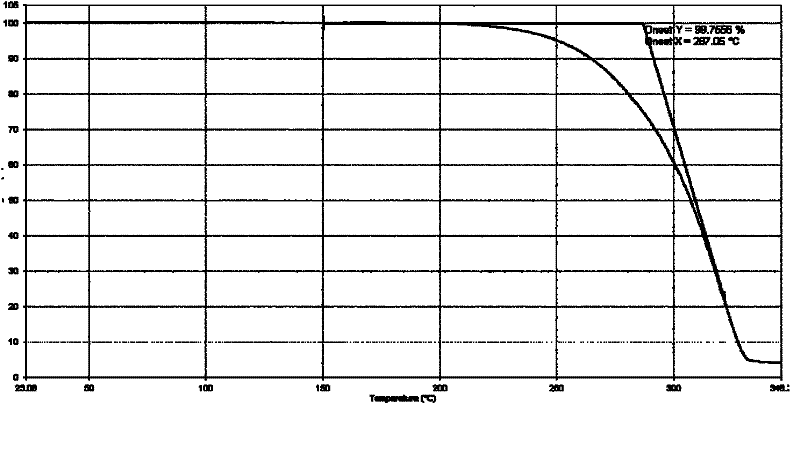
[0100] 50.0 g of abiraterone acetate (prepared according to the method provided in the literature organic preparations and procedures int., 29 (1), 123-134, 1997 examples) was added to the reaction flask, added to 250 ml of ethanol, and heated to reflux under stirring. After dissolving, add 250ml of water, stir for about 10 minutes, and then slowly cool down to 20-25°C to grow crystals for 1 hour. Suction filtration, the filter cake was rinsed with 50% ethanol aqueous solution. The filter cake was dried under reduced pressure at 50°C and -0.095MPa, and was assisted with phosphorus pentoxide. 46.2 g of white solid was obtained. Yield: 92.4%. Its typical XRPD pattern is as follows figure 1 shown.
[0101]
[0102] Scenario 2,
[0103] Add 5.0 g of abiraterone acetate into the reaction flask, add 30 ml of methanol, and heat up to reflux under stirring. After dissolving, add 30ml of water, stir for ab...
Embodiment 2 approach 1
[0109] Embodiment 2 scheme one: the prescription and preparation technology of abiraterone acetate preparation tablet:
[0110] The above abiraterone acetate polymorph I was formulated into tablets containing 250 mg with several excipients as follows.
[0111]
[0112]
[0113] The manufacture method of the tablet containing the abiraterone acetate polymorph I is to mix the first five kinds of the above-mentioned excipients with the abiraterone acetate polymorph I, add water in an appropriate amount to make a soft material, and make the soft material The wet granules are dried, the dried granules are evenly mixed with magnesium stearate and colloidal silicon dioxide, and then compressed to obtain abiraterone acetate tablets.
[0114] Scheme 2: Prescription and preparation process of abiraterone acetate capsules:
[0115] The above abiraterone acetate polymorph I was made into capsules containing 250 mg with several excipients as follows.
[0116]
[0117] The prepar...
Embodiment 2
[0118] The contents of the tablets and capsules prepared by the scheme of Example 2 are tested by X-ray powder diffraction, and the collection of graphs shows that the crystal form of the polymorph I that the bulk drug has is a characteristic peak with a 2θ relative intensity expressed in degrees exceeding 20%. All are completely displayed, which shows that the crystal form of the raw material drug remains consistent and has not changed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com