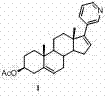
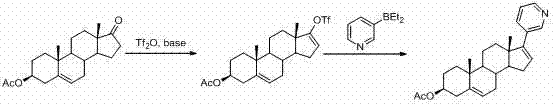
Method for preparing abiraterone acetate
A technology of abiraterone acetate and acetic acid, applied in the directions of steroids, organic chemistry, etc., can solve the problems of many by-products, long synthesis routes, and high cost of synthesis routes, and achieves few by-products, easy operation, and easy control of reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
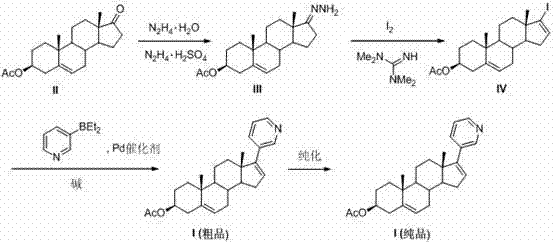
[0047] Example 1 and Example 2 respectively provide the preferred preparation methods of intermediate (III) and intermediate (IV). It can also be prepared with reference to other methods in the art.
[0048] Example 1 intermediate (Ⅲ)
[0049] Dehydroepiandrosterone (II) acetate (4.0 g, 12.1 mmol) and 60 mL of ethanol were placed in a 200 mL round bottom flask, then hydrazine hydrate (80%, 2.9 mL, 48.4 mmol) and hydrazine sulfate (15.6 mg, 0.12 mmol), the reaction was stirred at room temperature for 3 days. The raw materials were completely reacted, and then 100 mL of ice water was added, and a white solid was precipitated, filtered with suction, and the filter cake was washed with ice water (2×30 mL), Et 2 O (2×30 mL) was washed, and the product was dried in vacuo to finally obtain 3.54 g of intermediate (Ⅲ), with a yield of 84.8%. 1 H NMR (400 MHz, CDCl 3 ): δ 5.39 (d, J = 5.2 Hz, 1H), 4.75 (s, 2H), 4.60 (ddd, J = 10.4, 8.5, 4.2 Hz, 1H), 2.35-2.26 (m, 3H), 2.17 (dt,...
Embodiment 2
[0050] Example 2 intermediate (Ⅲ)
[0051] Dehydroepiandrosterone (II) acetate (40 g, 121 mmol) and 600 mL of ethanol were placed in a 1000 mL round bottom flask, then hydrazine hydrate (80%, 29 mL, 484 mmol) and hydrazine sulfate (156 mg, 1.2 mmol), stirred at room temperature for 3 days. The raw materials were completely reacted, and then 600 mL of ice water was added, and a white solid was precipitated, filtered with suction, and the filter cake was washed with ice water (2×300 mL), Et 2 O (2×300 mL) was washed, and the product was dried in vacuo to finally obtain 35.9 g of intermediate (Ⅲ), with a yield of 86.0%.
Embodiment 3
[0052] Example 3 Intermediate (Ⅳ)
[0053] I 2 (3.1 g, 12.2 mmol) dissolved in 100 mL THF and 48 mL Et 2 In the mixed solution of O, under nitrogen protection at 0°C, 1,1,3,3-tetramethylguanidine (1.8 mL, 14.6 mmol) was added, and after 5 min, the intermediate (Ⅲ) (2.0 g, 5.8 mmol dissolved in 48 mL THF), the drop was completed within 1 h, and the stirring reaction was continued for 1 h. The raw materials were completely reacted, filtered with suction, and the filtrate was spin-dried, then placed in an oil bath at 80°C and heated for 4 hours with stirring, cooled, and added 60 mL of Et 2 O, suction filtered, and the filtrate was washed with HCl (1M, 3×50 mL), anhydrous Na 2 SO 4 After drying, removing the solvent and recrystallizing (ethanol: water = 5: 1), finally 2.4 g of intermediate (Ⅳ) was obtained, with a yield of 94.2%. 1 H NMR (400 MHz, CDCl 3 ): δ 6.14 (dd, J = 3.1, 1.6 Hz, 1H), 5.38 (d, J = 5.2 Hz, 1H), 4.60 (ddd, J = 10.3, 8.4, 4.4 Hz, 1H), 2.39-2.30 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com