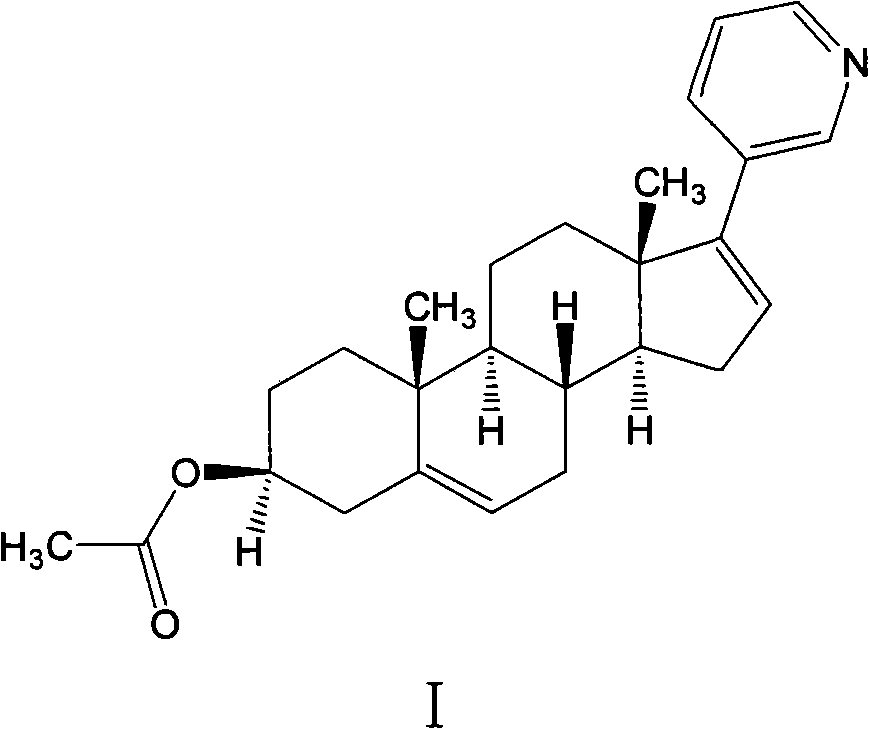
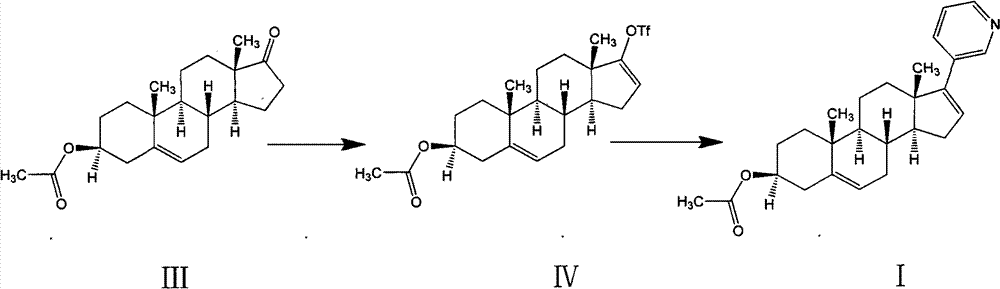
Preparation method of high-purity abiraterone acetate
A technology of abiraterone acetate and dehydroepiandrosterone acetate, which is applied in the field of preparation of abiraterone acetate and intermediates, can solve the difficulty of post-processing and production operations, the high price of trifluoromethanesulfonic acid, and unsuitability for industrialization No bad smell, low cost, easy to operate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 compound (IV) and abiraterone acetate crude product
[0046] (1) Preparation of compound (IV)
[0047]
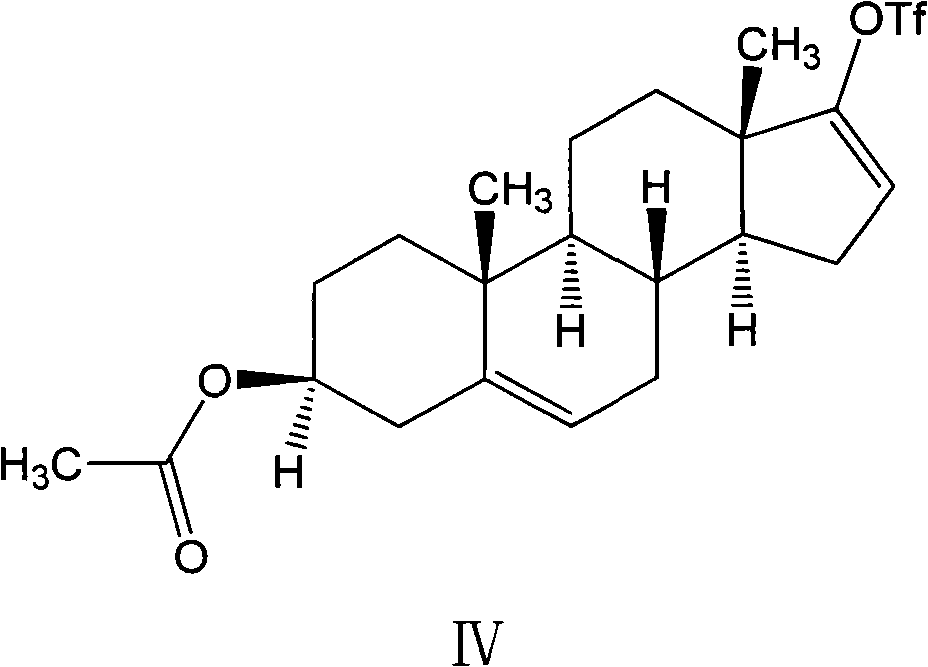
[0048] N 2 Under protection, after cooling 1.5L of dichloromethane to -10~-5°C, add 150g of dehydroepiandrosterone (III) acetate, 7.5g of dimethylaminopyridine (DMAP), 200g of Na 2 CO 3 . Add Tf slowly 2 O 180g, 1 ~ 1.5h drop, continue to react for 2 ~ 3h. Add 1.5 L of ice water to quench, separate the liquids, extract the aqueous phase with dichloromethane (0.5 L), combine the organic phases, wash 4 times with 1.5 L of water, dry over anhydrous sodium sulfate, and concentrate to obtain about 203 g of a purple-black oily compound ( IV), yield 96.6%.
[0049] (2) Preparation of Abiraterone Acetate Crude Product
[0050]
[0051] Formula IV compound (203g), THF (1.5L), Pd(PPh 3 ) 2 Cl 2 (2.7g), diethyl-(3-pyridine)borane (72g) and 2M Na 2 CO 3 (0.75L). Heat to an external temperature of 80°C, and react for 4 to 5 hours...
Embodiment 2~7
[0059] Embodiment 2~7: the preparation of compound (IV) and abiraterone acetate crude product
[0060] Use each material and consumption in Table 1 to obtain compound (IV) according to the method of embodiment 1 (1), then obtain the crude product of abiraterone acetate with reference to the method of embodiment 1 (2), wherein the consumption of each material can be adjusted appropriately according to the situation , the results are shown in Table 1.
[0061] Table 1:
[0062]
Embodiment 8
[0063] The preparation method of embodiment 8 high-purity abiraterone acetate
[0064]
[0065] Prepare the crude product of abiraterone acetate according to Example 1, take 100 g of the crude product of abiraterone acetate prepared in Example 1, add 400 ml of methanol, add 100 ml of 10% NaOH aqueous solution under stirring, stir for 4 hours, filter, and add 500 ml of dichloride to the filter cake Methane was heated to dissolve, washed twice with 200ml of water, dichloromethane was concentrated, and dried under reduced pressure at 50°C for 4h to obtain 78.5g of (II) as a solid.
[0066]
[0067] At room temperature, add 78.5g (II), 500ml of dichloromethane, and 35g of triethylamine, and slowly add acetyl chloride (the molar ratio of compound (II) is 4 equivalents) dropwise under stirring, and the dropwise addition is completed within 1 hour. The reaction was continued for 5 h. After the reaction was completed, 200 ml of water was added to wash 3 times. The organic phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com