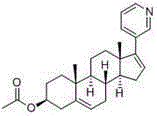
Abiraterone oral spray and use and preparation methods thereof
The technology of oral spray and abiraterone, which is applied in the field of pharmaceutical preparations, can solve problems such as difficulty in forming microemulsion, and achieve the effects of improving bioavailability and reducing the risk of toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] An oral spray of abiraterone, comprising the following components by weight percentage, abiraterone acetate 0.44%; oil phase (medium chain triglyceride, ethyl oleate or oleic acid) 9.05%; Co-surfactant (ethanol, n-butanol or propylene glycol) 4.42%; surfactant (polyoxyethylene 40 hydrogenated castor oil or polyoxyethylene 35 castor oil) 19.18%; balance of water.
[0040] A preparation method of abiraterone oral spray, specifically comprising the following steps:
[0041] (1) Weigh the oil phase, co-surfactant, and surfactant of the formula respectively and stir them evenly;
[0042] (2) Weigh the formula amount of abiraterone acetate and stir in it, and then place it in a water bath at 40-50°C;
[0043] (3) Stir the mixture in step (2) at a speed of 210rpm for 30 minutes to form a microemulsion;
[0044] (4) Cool the microemulsion in step (3) naturally, filter and fill it in a spray bottle with a quantitative valve to prepare the oral spray of abiraterone acetate. The t...
Embodiment 2
[0046] An oral spray of abiraterone, by weight percentage, comprising the following components, abiraterone acetate 2.17%; oil phase (medium chain triglyceride, ethyl oleate or oleic acid) 5.01%; Co-surfactant (ethanol, n-butanol or propylene glycol) 5.46%; surfactant (polyoxyethylene 40 hydrogenated castor oil or polyoxyethylene 35 castor oil) 11.07%; balance of water.
[0047] A preparation method of abiraterone oral spray, specifically comprising the following steps:
[0048] (1) Weigh the oil phase, co-surfactant, and surfactant of the formula respectively and stir them evenly;
[0049] (2) Weigh the formula amount of abiraterone acetate and stir in it, and then place it in a water bath at 40-50°C;
[0050] (3) Stir the mixture in step (2) at a speed of 210rpm for 30 minutes to form a microemulsion;
[0051] (4) Cool the microemulsion in step (3) naturally, filter and fill it in a spray bottle with a quantitative valve to prepare the oral spray of abiraterone acetate. Th...
Embodiment 3
[0053] A kind of oral spray of abiraterone, by weight percentage, comprises following component, abiraterone acetate 0.51%; Abiraterone acetate is 3 / 50 of oily phase, and oily phase (medium chain triglyceride ester, ethyl oleate or oleic acid) specifically 8.5%; co-surfactant (ethanol, n-butanol or propylene glycol) co-surfactant is 1 / 3 of the surfactant, specifically 4.56%; surfactant (polyoxyethylene 40 hydrogenated castor oil or polyoxyethylene 35 castor oil) 13.68%; flavoring agent 2.65% (sucrose 1.77%, banana powder 0.88%); antibacterial agent potassium sorbate 0.19%; water balance.
[0054] A preparation method of abiraterone oral spray, specifically comprising the following steps:
[0055] (1) Weigh the oil phase, co-surfactant, and surfactant of the formula respectively and stir them evenly;
[0056] (2) Weigh the formula amount of abiraterone acetate and stir in it, and then place it in a water bath at 40-50°C;
[0057] (3) First, dissolve the flavoring agent and po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com