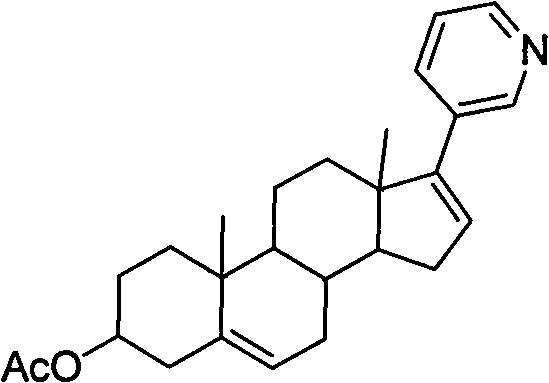
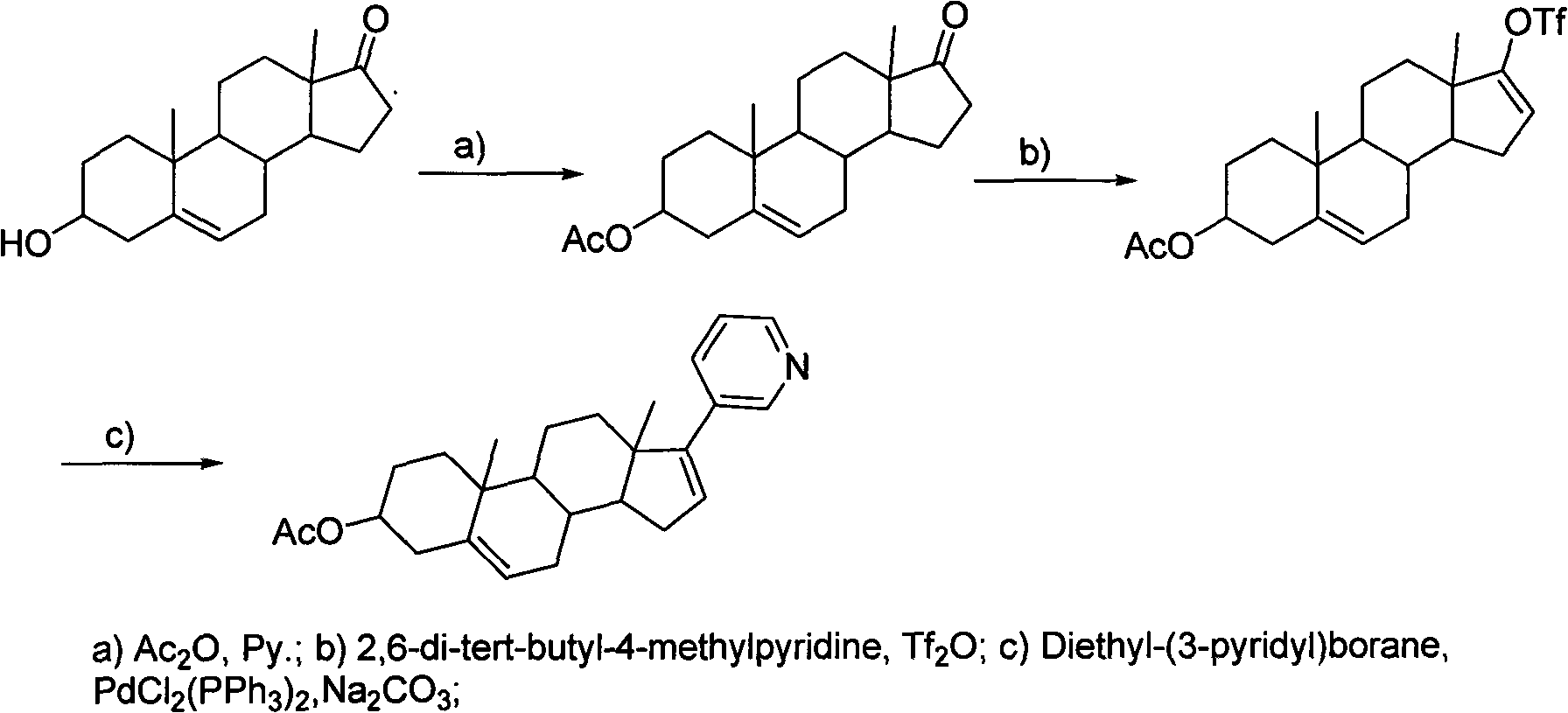
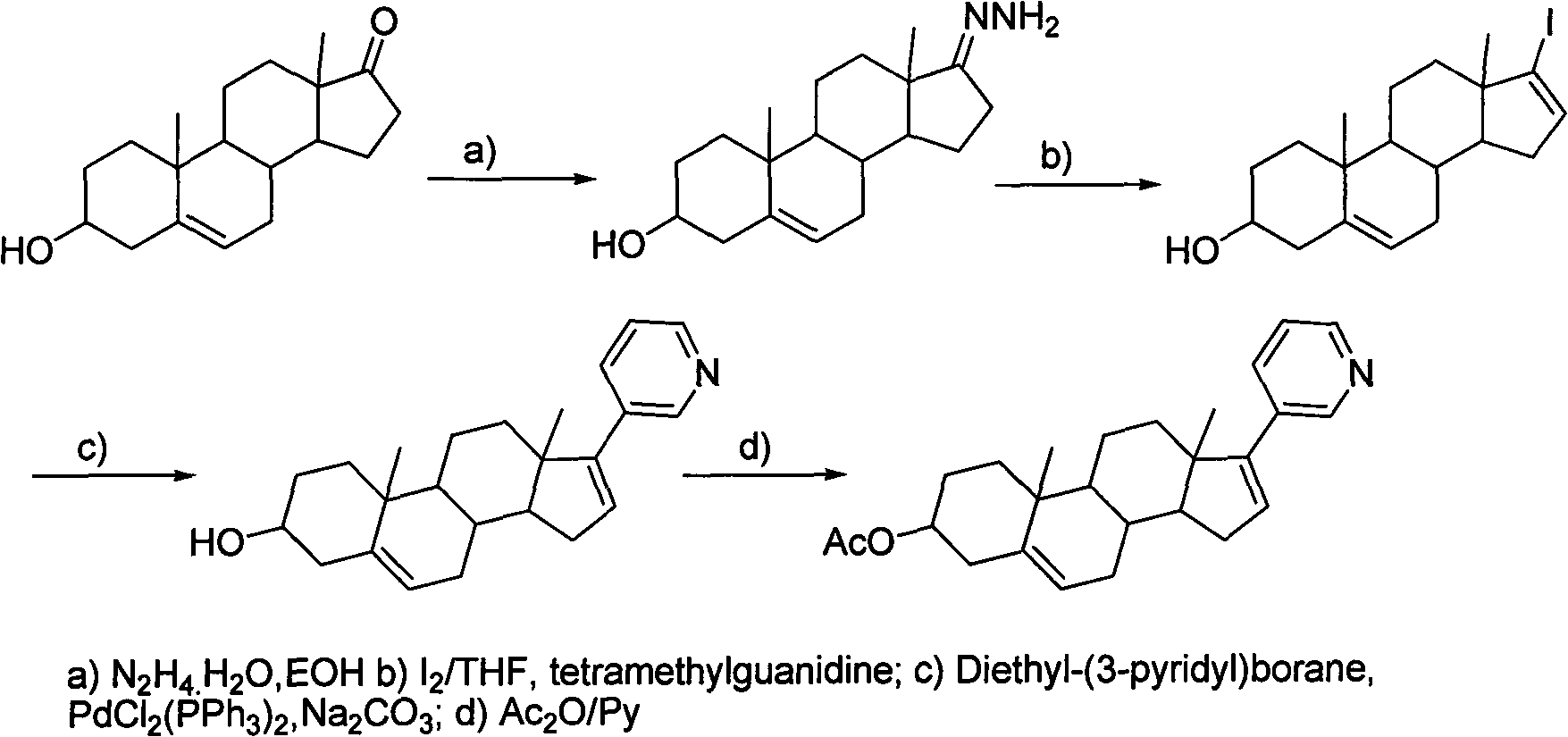
Novel synthesis method of Abiraterone acetate
A technology of abiraterone acetate and a new method, applied in the direction of steroids, organic chemistry, etc., can solve the problems of inconvenient transportation, high market price, flammability and explosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 17-(3-pyridyl)androst-5,16-dien-3beta-ol
[0032] Add 750ml of THF to a 3L three-necked flask, and add 50g of 17-iodo-androst-5,16-dien-3beta-ol, 264mg of bistriphenylphosphinepalladium chloride and 16.44g of pyridine-3 - boric acid, finally add 345ml 2mol / L Na 2 CO 3 solution. Heat to reflux, control the internal temperature at 85-90°C, and monitor the completion of the reaction by TLC. Cool the reaction solution to room temperature, add 1500ml of water to the reaction solution, stir for solid precipitation, stir for 30min, and filter. The filter cake was stirred and washed 4 times with water. The filter cake was air-dried at 50-55°C overnight to obtain 41.9 g of solid.
Embodiment 2
[0034] 17-(3-pyridyl)androst-5,16-dien-3beta-ol
[0035] Add 750ml of THF to a 3L three-necked flask, and add 44.1g of 17-bromo-androst-5,16-dien-3beta-ol, 264mg of bistriphenylphosphinepalladium chloride and 16.44g of pyridine-3 - boric acid, finally add 345ml 2mol / L Na 2 CO 3 solution. Heat to reflux, control the internal temperature at 85-90°C, and monitor the completion of the reaction by TLC. Cool the reaction solution to room temperature, add 1500ml of water to the reaction solution, stir for solid precipitation, stir for 30min, and filter. The filter cake was stirred and washed 4 times with water. The filter cake was air-dried overnight at 50-55° C. to obtain 39.4 g of solid.
Embodiment 3
[0037] 17-(3-pyridyl)androst-5,16-dien-3beta-ol
[0038] Add 750ml of THF to a 3L three-necked flask, and add 52.8 grams of 17-trifluoromethanesulfonate-androst-5,16-diene-3beta-ol and 264mg of bistriphenylphosphine palladium chloride successively under stirring And 16.44 grams of pyridine-3-boronic acid, finally add 345ml 2mol / L Na2CO3 solution. Heat to reflux, control the internal temperature at 85-90°C, and monitor the completion of the reaction by TLC. Cool the reaction solution to room temperature, add 1500ml of water to the reaction solution, stir for solid precipitation, stir for 30min, and filter. The filter cake was stirred and washed 4 times with water. The filter cake was air-dried at 50-55°C overnight to obtain 43.5 g of solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com