Purification method of abiraterone
A technology of abiraterone and purification method, which is applied in the fields of organic chemistry and steroids, and can solve the problems of high cost, time-consuming, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
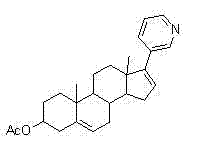
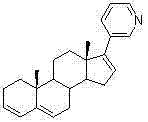
[0030] Preparation of 17-(3-pyridyl)androst-5,16-dien-3beta-ol (abiraterone)
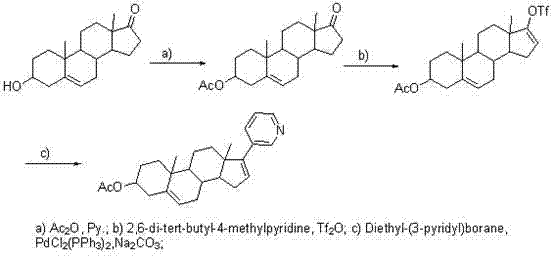
[0031] Add 750ml of THF to a 3L three-necked flask, and add 50g of 17-iodo-androst-5,16-dien-3beta-ol, 264mg of bistriphenylphosphinepalladium chloride and 16.44g of pyridine-3-di Ethylborane, finally add 345ml 2mol / L Na 2 CO 3 solution. Heat to reflux, control the internal temperature at 85-90°C, and monitor the completion of the reaction by TLC. Cool the reaction solution to room temperature, add 1500ml of water to the reaction solution, stir for solid precipitation, stir for 30min, and filter. The filter cake was stirred and washed 4 times with water. The filter cake was air-dried overnight at 50-55° C. to obtain 26.3 g of crude abiraterone.
Embodiment 2
[0033] Purification of 17-(3-pyridyl)androst-5,16-dien-3beta-ol (abiraterone)
[0034] Add 34.9g of Abiraterone to 350ml of THF at room temperature, and stir until dissolved. Under an ice bath, cool to 0-5°C, add 4.9g of concentrated sulfuric acid dropwise, and control the internal temperature not to exceed 10°C. After the dropwise addition, continue to stir in an ice bath for 30 min, filter, stir and wash with pre-cooled THF (50ml×2), and drain to obtain the crude abiraterone sulfate. Add the obtained abiraterone sulfate to 200ml of ethanol / water (4:1) mixed solvent, heat to reflux to dissolve, slowly cool to room temperature, add ice bath, and stir for 2h. Filter and wash with pre-cooled ethanol (40ml×2). After draining, dissolve the solid in 100ml of water, add 40% NaOH solution dropwise under ice bath, and adjust the pH to 8-9. Filter and wash with pure water (40ml×2). Blow drying at 50-60°C to obtain 32.8g of abiraterone, HPLC: 99.6%, single impurity <0.1%.
Embodiment 3
[0036] Purification of 17-(3-pyridyl)androst-5,16-dien-3beta-ol (abiraterone)
[0037] Add 34.9g of Abiraterone to 350ml of THF at room temperature, and stir until dissolved. Under an ice bath, cool to 0-5°C, add 11.4g of trifluoroacetic acid dropwise, and control the internal temperature not to exceed 10°C. After the dropwise addition was completed, continue to stir in an ice bath for 30 minutes, filter, stir and wash with pre-cooled THF (50ml×2), and drain to obtain the crude product of abiraterone trifluoroacetate. Add the obtained abiraterone trifluoroacetate into 200ml of ethanol / water (4:1) mixed solvent, heat to reflux to dissolve, slowly cool to room temperature, add ice bath, and stir for 2h. Filter and wash with pre-cooled ethanol (40ml×2). After draining, dissolve the solid in 100ml of water, add 40% NaOH solution dropwise under ice bath, and adjust the pH to 8-9. Filter and wash with pure water (40ml×2). Blow drying at 50-60°C to obtain 33.1 g of abiraterone, H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com