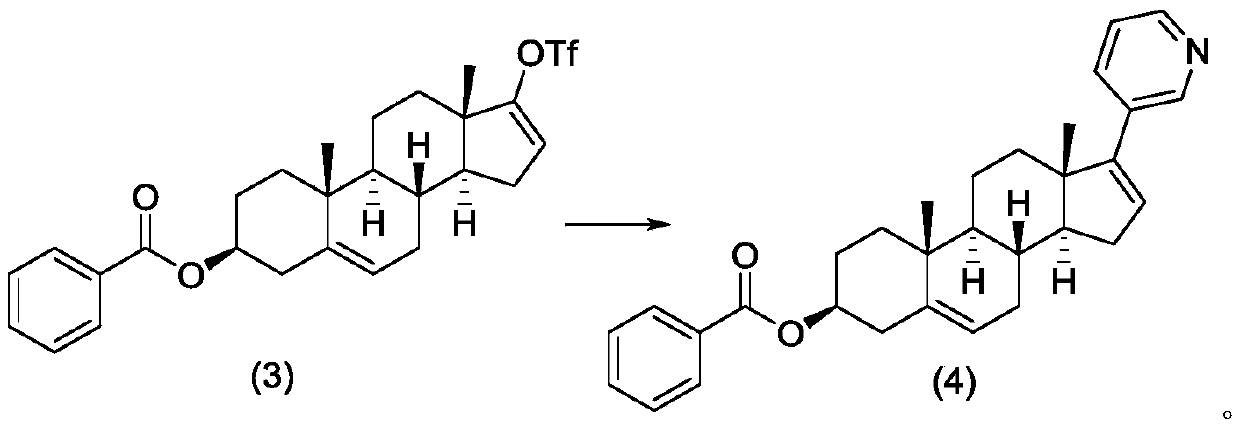
Method for preparing abiraterone acetate
A technology of abiraterone acetate and abiraterone, applied in the direction of steroids, organic chemistry, etc., can solve the problems of failure to show large-scale production, yield and conversion rate need to be further improved, poor performance in yield, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
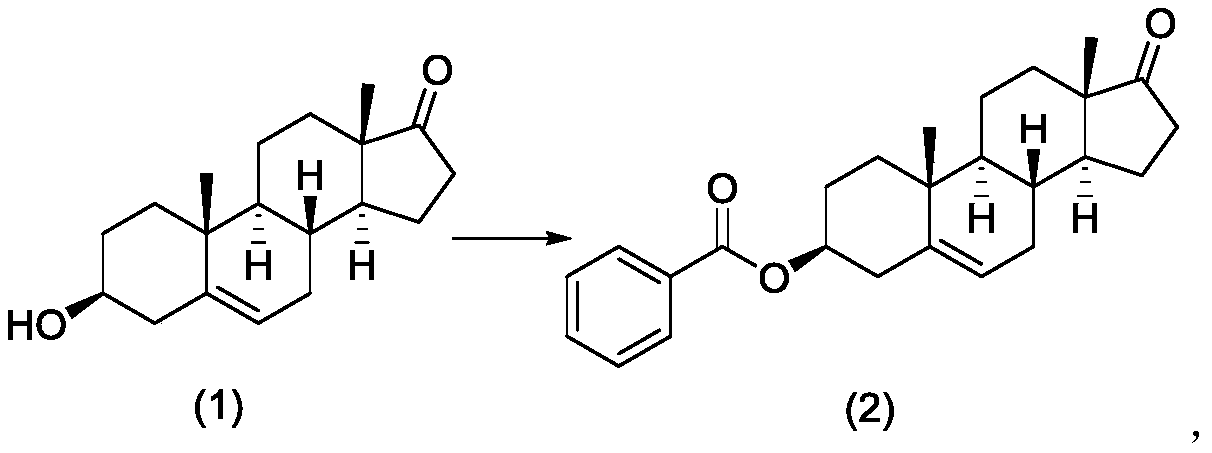
[0105] Add dehydroepiandrosterone (1) (150.00 g, 0.52 mol) and pyridine (750.00 g) sequentially into a 1000 mL three-necked flask, and stir to dissolve. Under the ice bath, control the temperature at 10-25°C, add benzoyl chloride (109.66g, 0.78mol) dropwise, remove the ice bath after dropping, stir until the reaction is complete, add water to quench the reaction, filter, filter the cake with water (150mL x 2 ), rinsed with air, and air-dried at 50° C. to obtain compound (2) as a white solid. The yield was 200.01 g, the yield was 98.0%, and the purity: 100% (HPLC).
[0106]
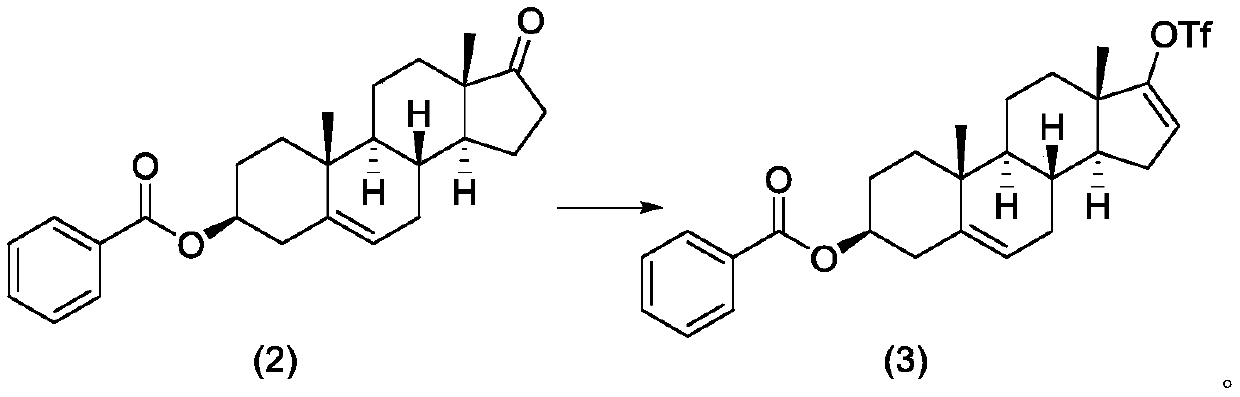
[0107] Compound (2) (100.00 g, 0.255 mol) and dichloromethane (2000 mL) were sequentially added into a 3000 mL three-neck flask, and cooled in an ice-water bath to obtain a white suspension. Under the protection of nitrogen, the temperature is controlled at -5 ~ 5°C, and trifluoromethanesulfonic anhydride (100.62g, 0.357mol) and triethylamine (28.36g, 0.280mol) are added dropwise in sequenc...
Embodiment 2
[0109] Embodiment 2: optimization of benzene methyl sulfonation conditions
[0110]
[0111] With reference to the operating process in Example 1 step 1, factors such as the types of benzoyl chloride consumption, alkali and solvent are investigated respectively in the reaction to obtain optimal process conditions
[0112] Numbering Dosage of Benzoyl Chloride base solvent yield 1 1.5 equivalent Triethylamine / DMAP Dichloromethane 74.9% 2 1.5 equivalent pyridine NA 97.7%
Embodiment 3
[0114]
[0115] With reference to the operation process in step 2 of Example 1, factors such as the types of alkali and solvent in the reaction are investigated respectively to obtain optimal process conditions
[0116] Numbering Dosage of trifluoromethanesulfonic anhydride base temperature reflex yield 1 1.5 equivalent NMM -15~5℃ 92.8% 2 1.5 equivalent pyridine -15~5℃ 93.0% 3 1.5 equivalent Ac 2 O / AcOH / K 2 CO 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com