Abiraterone derivative with anti-cancer effect
A technology of abiraterone and its derivatives, which is applied in the field of abiraterone derivatives, can solve problems such as difficulties in the form of prodrugs, and achieve the effect of improving solubility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
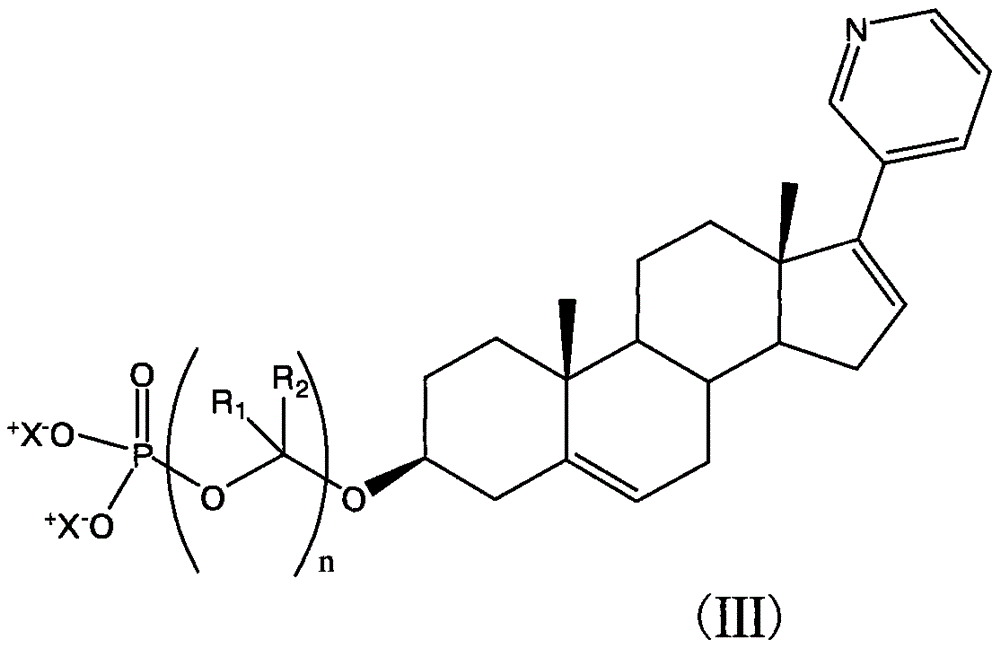
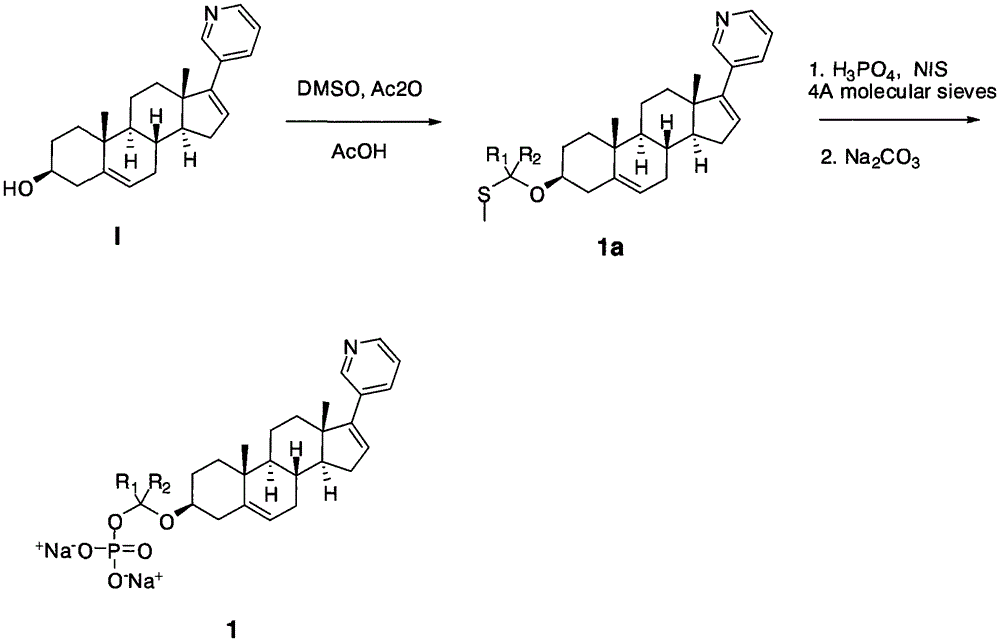
[0034] The compounds shown can be conveniently prepared using known organic synthesis techniques. Many specific preparation methods are published in references well known to those skilled in the art. One of the representative documents is March 1994, Advanced Organic Chemistry, Reactions, Mechanis and Structure, N.Y, McGraw Hi ll. This embodiment is specifically a synthesis step of the following structure:
[0035]
[0036] The first step: Raw material I (109 mg, 0.31 mmol) is dissolved in a mixture of acetic acid (5 mL), acetic anhydride (1.0 mL, 10.5 mmol) and DMSO (1.5 mL). The reaction was stirred at room temperature for 5 days, poured into 100 mL of water, neutralized with sodium bicarbonate, and extracted with ethyl acetate. The separated organic phase was dried with anhydrous sodium sulfate, then filtered and concentrated. It was separated by column chromatography on silica gel (hexane / ethyl acetate 2 / 1) to obtain product 1a (65 mg, yield 50%); diluted with ethyl acetate...
Embodiment 2
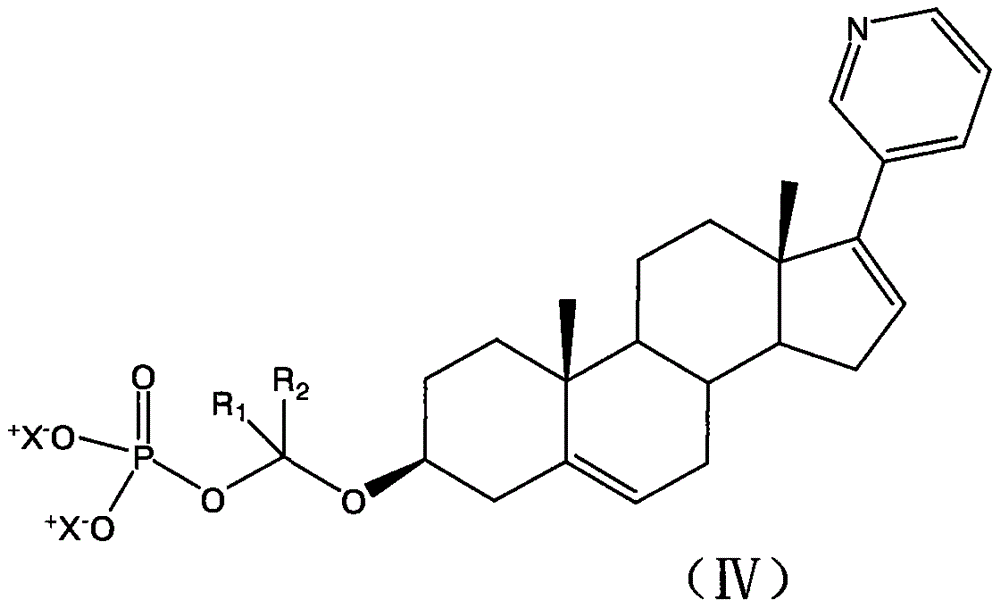
[0039] This example has similar synthesis steps as in Example 1. The steps for synthesizing the following structure are known to those skilled in the art to synthesize the following compounds by changing the starting materials and reaction conditions.
[0040]
[0041] (2)
Embodiment 3
[0043] This example has similar synthesis steps as in Example 1. The steps for synthesizing the following structure are known to those skilled in the art to synthesize the following compounds by changing the starting materials and reaction conditions.
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com