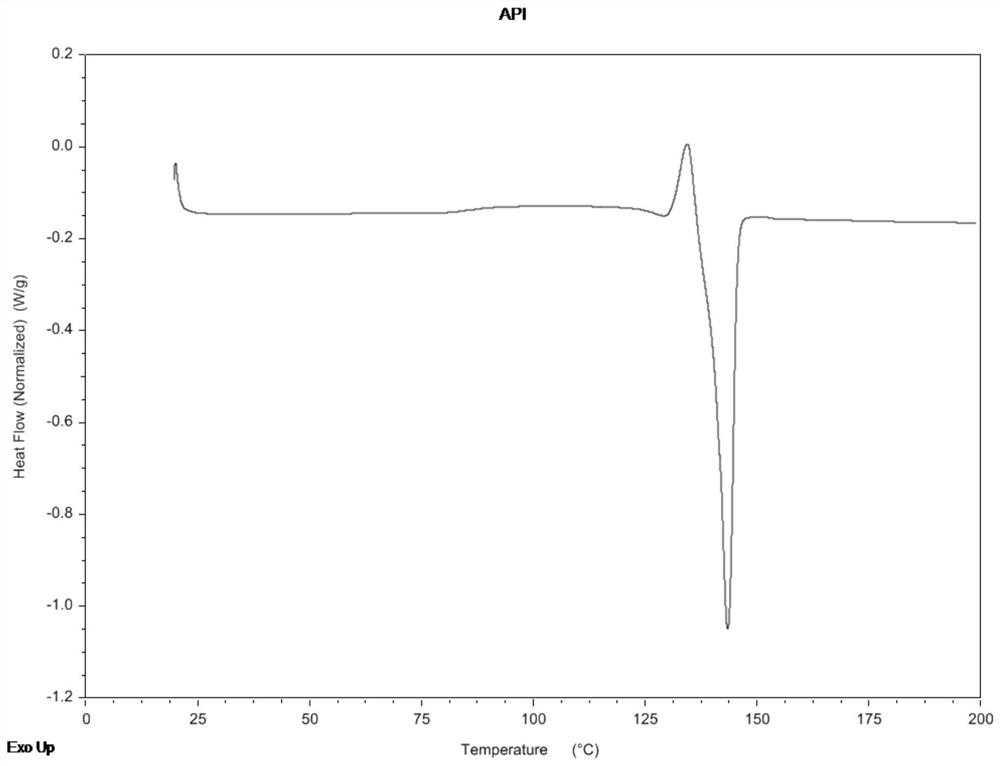
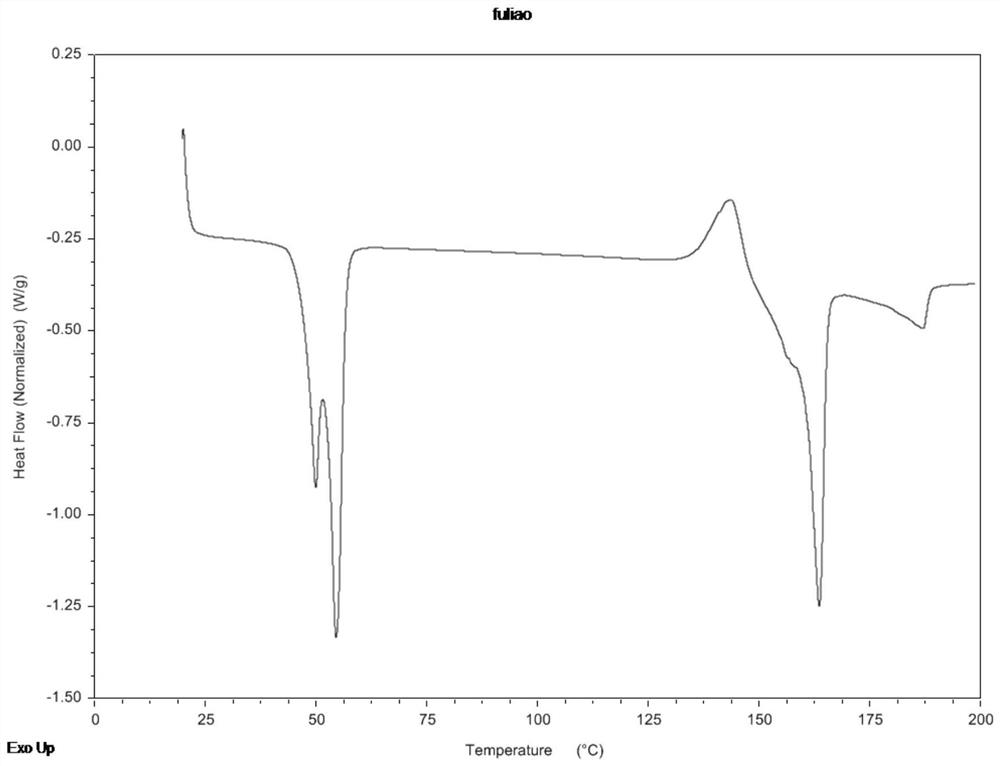
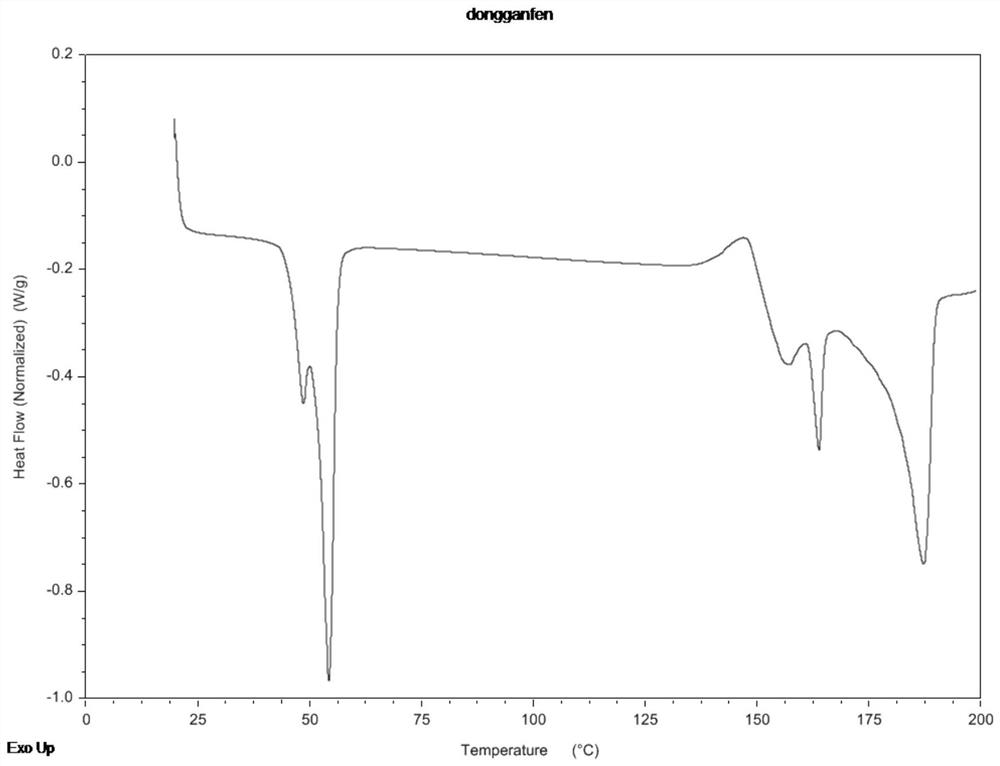
Abiraterone acetate nanocrystal as well as preparation and preparation method thereof
A technology of abiraterone acetate and nanocrystals, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of increased absorption and low bioavailability, and achieve increased absorption. , The effect of reducing drug cost, improving solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 screens the kind of stabilizer
[0030] Preparation method: First, weigh the abiraterone acetate raw material and stabilizer at a ratio of 5:1, add appropriate amount of water, and ultrasonically mix the solution evenly, then perform high-speed shearing on the solution, remove the initial suspension of the lower layer, add zirconia grinding beads and A stir bar was used to obtain nanocrystals of abiraterone acetate after media grinding. The obtained nanocrystals were mixed uniformly with a lyoprotectant (sucrose and mannitol in a weight ratio of 1:1, and the total dosage was 10% w / v), and then vacuum-dried for 48 hours to obtain a lyophilized powder of abiraterone acetate nanocrystal preparation.
[0031] Sodium dodecyl sulfate (SDS), hydroxypropyl methylcellulose (HPMC), poloxamer 188 (P188), poloxamer 407 (P407), polyvinylpyrrolidone K30 (PVP K30) and spit Temperature 80 (Tween 80) six stabilizers. Through detection, it is found that the nanocrystal con...
Embodiment 2
[0032] The screening of the ratio of embodiment 2 medicines and stabilizer
[0033] Adjust the weight ratio of abiraterone acetate bulk drug and stabilizer (select poloxamer 407 or poloxamer 188), and other preparation methods are the same as in Example 1.
[0034] Screened abiraterone acetate bulk drug: three weight ratios of stabilizer P407=3:1, 4:1, 5:1, and abiraterone acetate bulk drug: stabilizer P188=3:1, 4:1, 5:1 three weight ratios, it is found that different stabilizers are prepared according to the ratio of 5:1, the particle size of the preparation is smaller and the stability is the best.
Embodiment 3
[0035] Embodiment 3 is to the screening of each stabilizer ratio of compound stabilizer
[0036] According to the screening of stabilizers in Example 1, two stabilizers, Poloxamer 407 and Poloxamer 188, were considered for compounding. A total of four ratios of P407:P188=7:1, 1:1, 3:5, and 1:3 were screened, and it was found that the formulation of 7:1 had a smaller particle size and the best stability. The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com