Method for preparing medical composition of abiraterone or derivative thereof, and application of medical composition
An abiraterone and a technology for preparing medicines, which are applied in the field of pharmaceutical preparations and can solve problems such as differences in poor bioavailability between individuals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
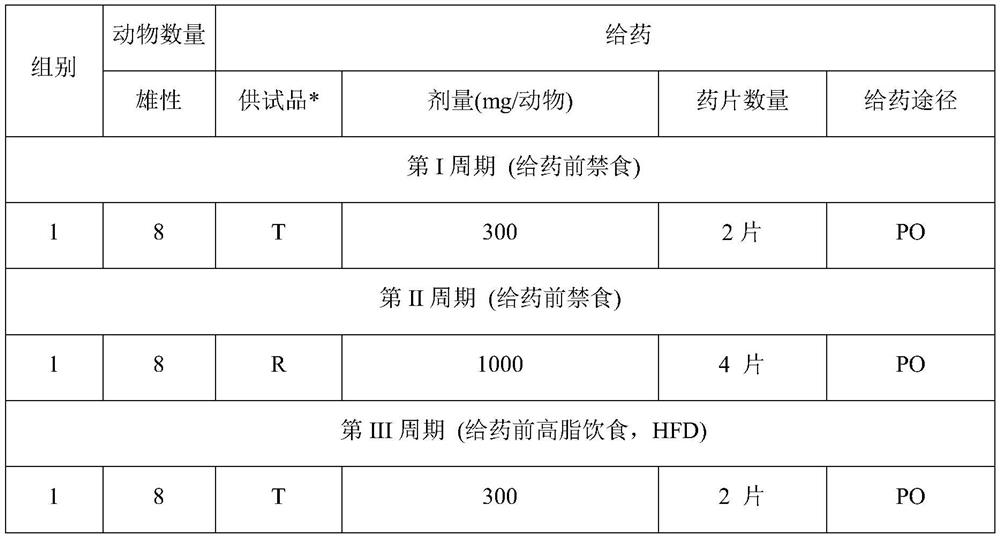
Examples
Embodiment 1
[0101]
[0102] 1) Preparation of nanosuspension
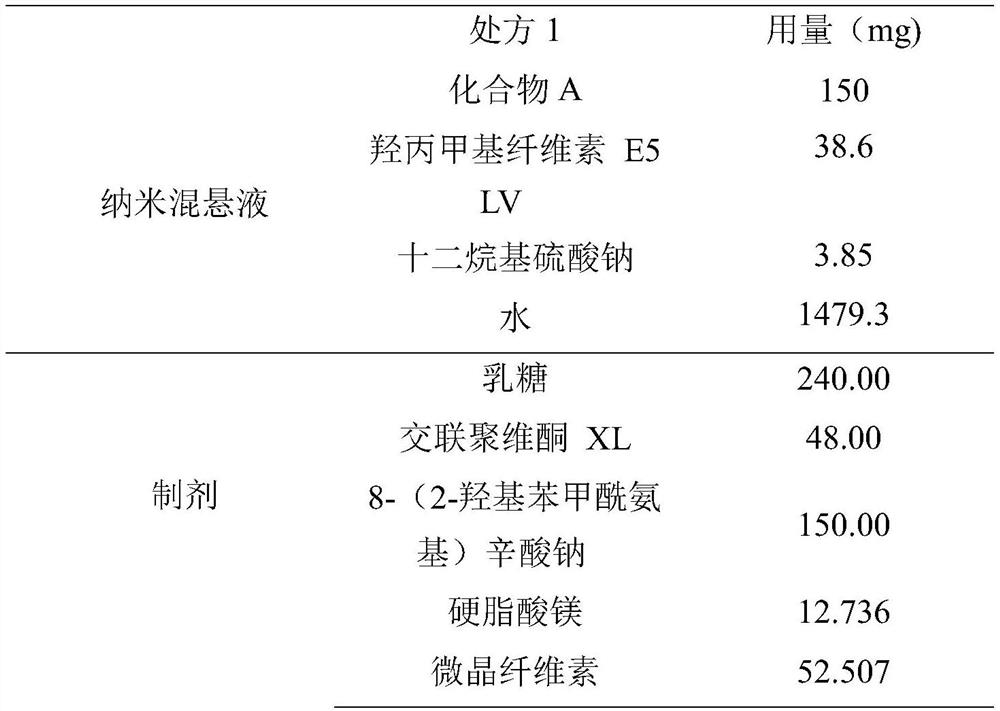
[0103] Add 19.2g of HPMC E5 LV to 739.6g of water to disperse and dissolve, then add 1.92g of SDS to dissolve, then add 75g of compound A to the above solution, stir to disperse.
[0104] Sand mill is installed (grinding chamber volume is 160ml, fills the 0.3mm grinding bead of 112ml, and feed rate is 160rpm, and grinding speed is 3000rpm), and dispersed suspension is added into grinder preparation tank, starts stirring, grinds to obtain Compound A nanosuspension D90 500nm, for future use.
[0105] 2) Fluidized bed granulation
[0106] Take 1003 g of the above-mentioned nano-suspension, add 90 g of the absorption-promoting agent SNAC into the above-mentioned nano-suspension, and stir and disperse. Add 144g of lactose and 28.8g of crospovidone XL into the fluidized bed for fluidized bed top spray granulation. After the granulation is completed, the granules are dried, and the drying can be stopped when the water content o...
Embodiment 2
[0115]
[0116] 1) Preparation of nanosuspension
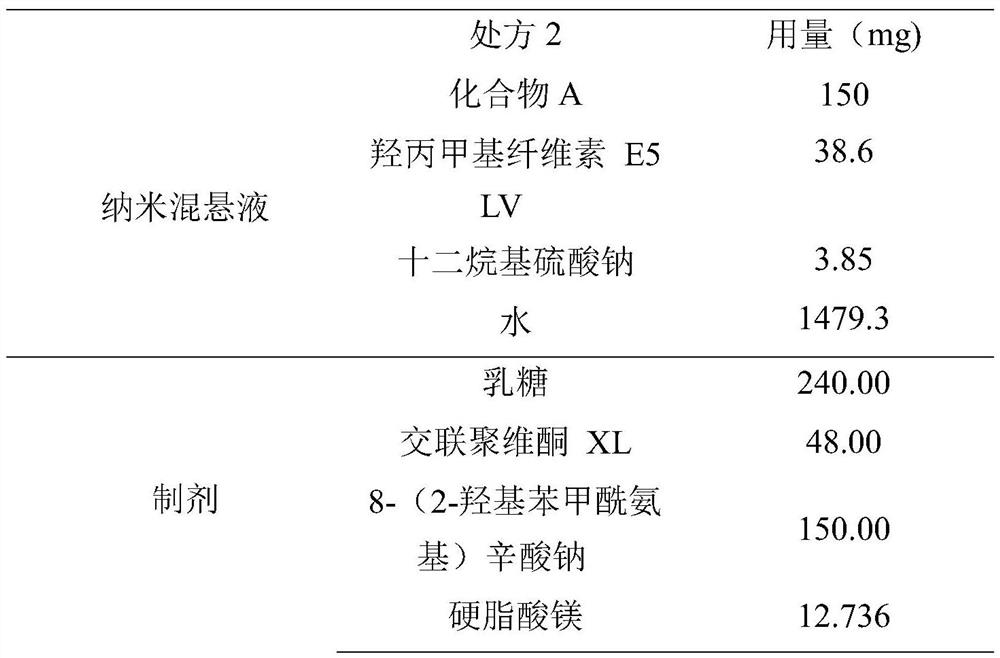
[0117] Add 19.2g of HPMC E5 LV to 739.6g of water to disperse and dissolve, then add 1.92g of SDS to dissolve, then add 75g of compound A to the above solution, stir and disperse.
[0118] Sand mill is installed (grinding chamber volume is 160ml, fills the 0.3mm grinding bead of 112ml, and feed rate is 160rpm, and grinding speed is 3000rpm), and dispersed suspension is added into grinder preparation tank, starts stirring, grinds to obtain Compound A nanosuspension D90 is about 500nm, for future use.
[0119] 2) Fluidized bed granulation
[0120] Take 1170.2 g of the above-mentioned nano-suspension, add 105 g of the absorption-promoting agent SNAC into the above-mentioned nano-suspension, and stir and disperse. Add 168g of lactose and 33.6g of crospovidone XL into the fluidized bed for fluidized bed top spray granulation. After the granulation is completed, the granules are dried, and the drying can be stopped when the wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com