Method for quantitatively detecting abiraterone in whole blood
A quantitative detection method, abiraterone technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that it is difficult to find out the content of abiraterone in whole blood, and the content of abiraterone has not been known, and achieve the improvement of sensitivity , Strong elution ability, fast elution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
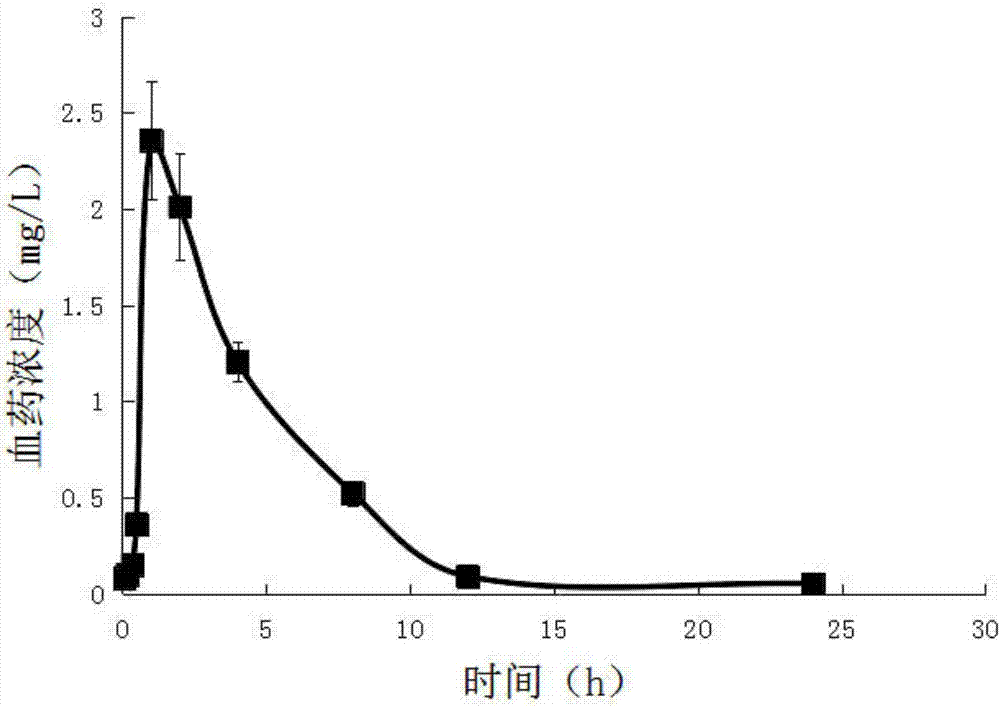
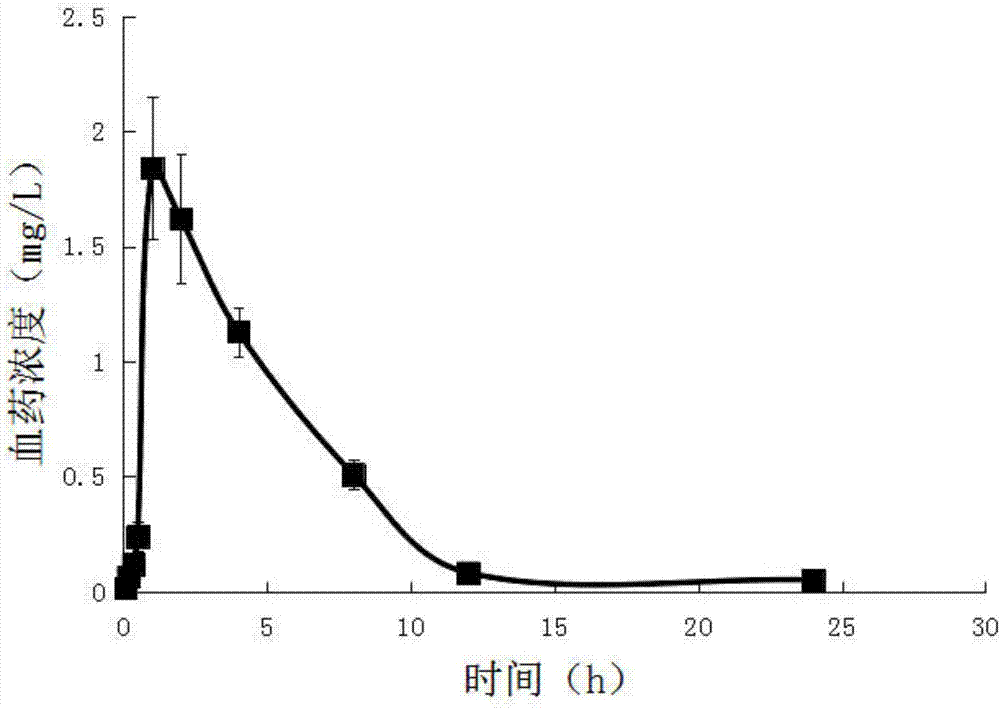
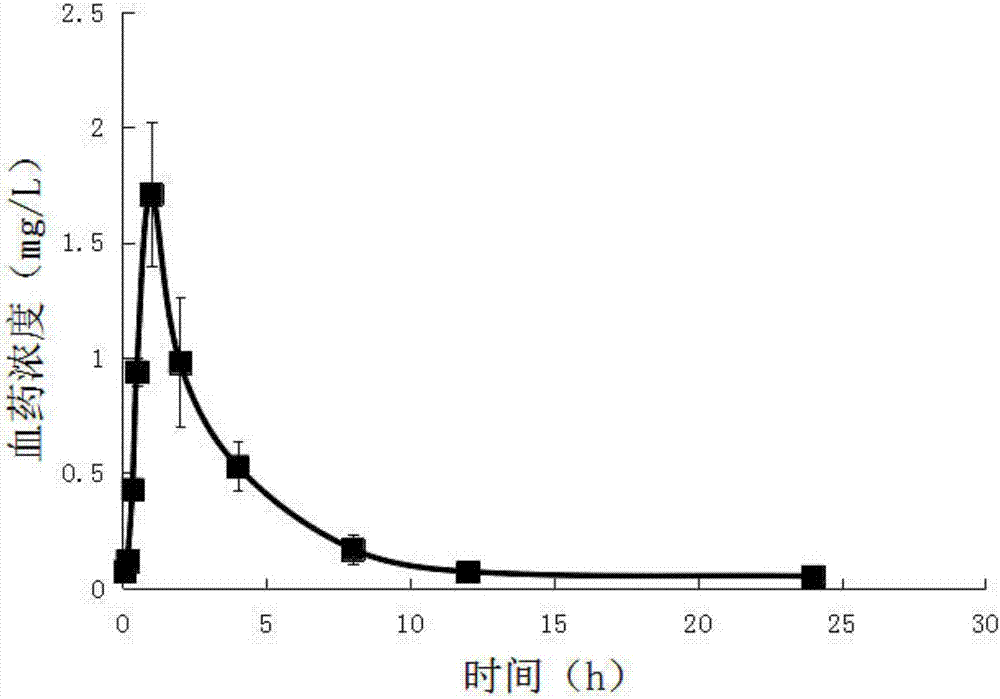
[0047] Embodiment 1, a kind of abiraterone pretreatment method and quantitative detection means in animal (human) whole blood, carry out following steps successively:
[0048] 1) Put 0.2mL of venous whole blood separated from the animal body / human body into a heparinized centrifuge tube, add 0.2mL of cell lysate, and shake (shake at a frequency of 100r / min for 5 minutes) to completely lyse the blood cells; Whole blood lysate.
[0049] The formula of the above-mentioned cell lysate is: 8.29g of ammonium chloride, 1g of potassium bicarbonate and 37.2mg of EDTA-2Na, which are dissolved in 1000mL of ultrapure water and stirred to dissolve.
[0050] Accurately draw 0.4 mL of whole blood lysate into a centrifuge tube with a cover. After shaking and mixing, add 3 mL of ethyl acetate to it accurately, vortex (100 r / min frequency) for 2 min, and centrifuge at 8000 r / min for 5 min to obtain Ethyl acetate layer in the upper layer, residue in the lower layer. Take out all the ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com