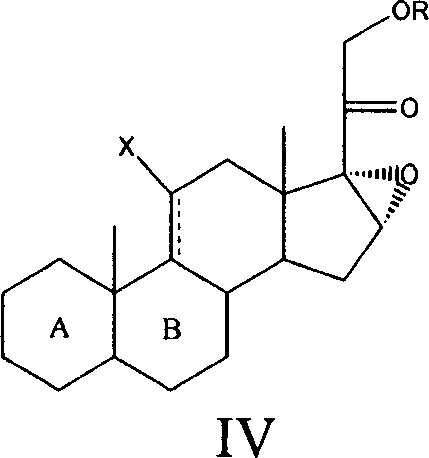
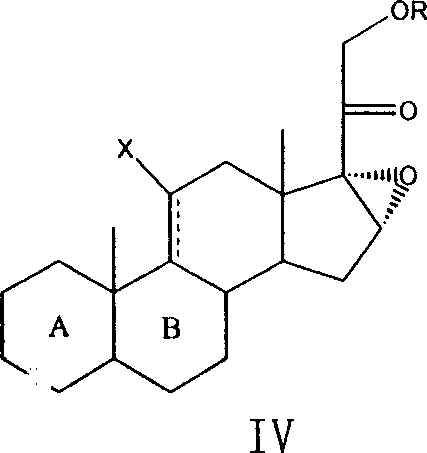
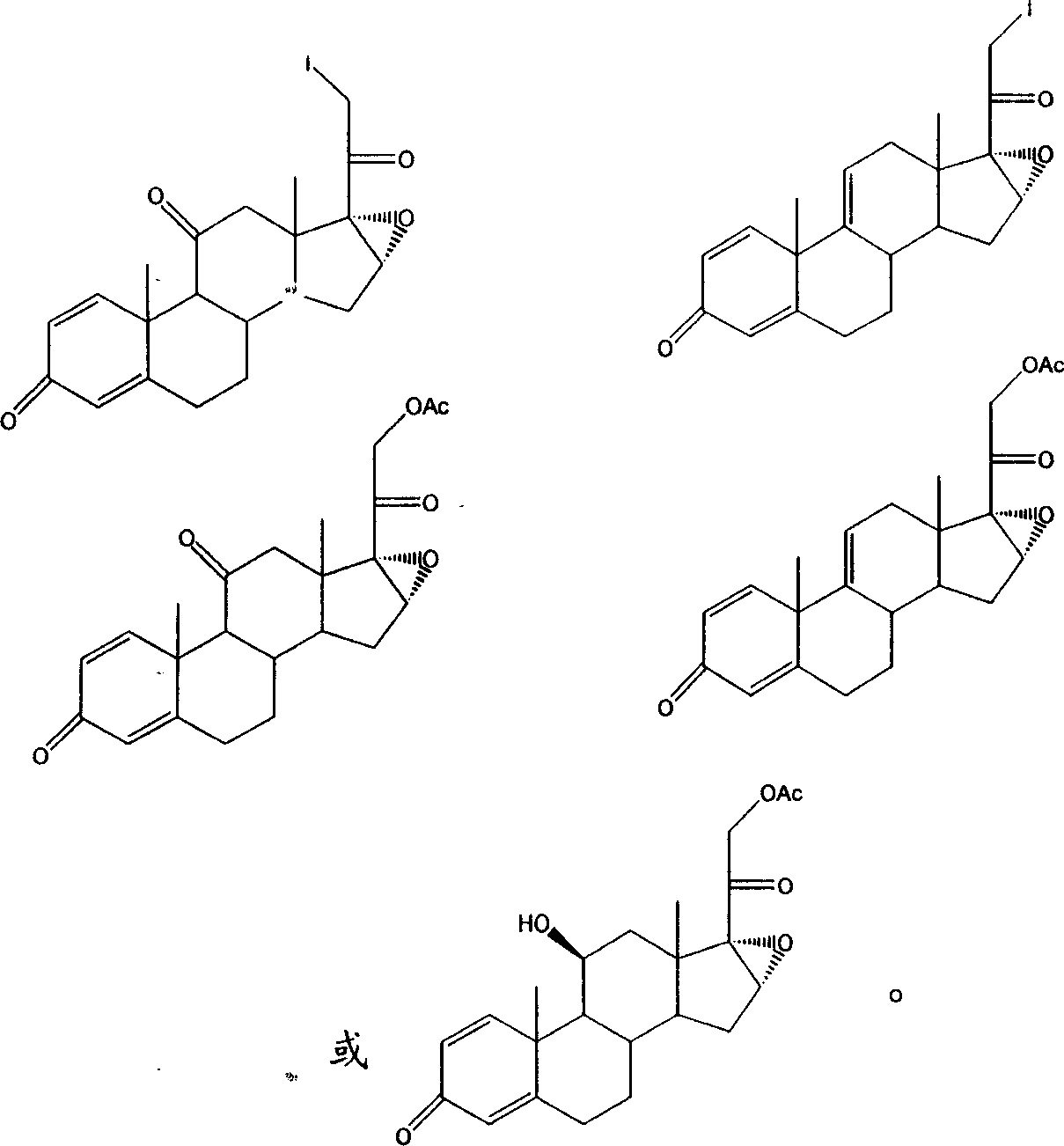
Method for preparing steroids and novel intermediate compound used therein
A compound and Chinese-style technology, applied in the field of pine preparation, can solve the problems of long production cycle, reduced yield of final product, and increased impurity content of final product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 30ml of methanol and 8 grams of iodine were stirred to make iodine solution for later use. Put 60ml of tetrahydrofuran, 6g of calcium oxide, and 10g of Sml into the reaction flask, stir for 10 minutes, then slowly add iodine solution dropwise at 15-20°C, stir for 3 hours after the addition, dilute in 10% acetic acid / water (V / V) 500ml, after stirring for 1 hour, filter to obtain Sm2. mp: 132-140°C. Its infrared data is as follows:
[0085] 3000-2800, 1728, 1658, 1630, 1620, 1475-1400, 1372-1300, 1155cm -1 .
Embodiment 2
[0087] Put 60ml of acetone and Sm2 into the reaction flask, stir for 10 minutes, add 8 grams of potassium acetate and heat up to 60-70°C, keep it warm for 4 hours, then reduce to 40°C and concentrate under reduced pressure. Dilute in 500ml of water after a large number of crystals appear, and stir After 1 hour, it was filtered, washed with water until neutral, and dried to obtain 10.53 g of Sm3 solid. mp: 205-209°C. Its infrared data is as follows:
[0088] 3000-2800, 1746, 1728, 1658, 1630, 1620, 1475-1400, 1372-1300, 1155cm -1 .
Embodiment 3
[0090] Add 30ml of 50-55% hydrogen bromide / water (V / V) solution and 30ml of acetic acid into the reaction flask, cool down to 0°C and slowly add 10g of Sm3 under stirring, after the addition, keep warm at 10°C for 3 hours, after the reaction is completed, The reaction solution was diluted in 600ml of water, washed with water until neutral and dried to obtain Sm4 for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com