Whole blood type freeze-dried powder immunosuppressant quality control substance as well as preparation method and application thereof
An immunosuppressant, freeze-dried powder technology, applied in the field of medical testing, can solve the problems of unsuitable long-term storage, lack of ultra-low temperature storage devices, etc., and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
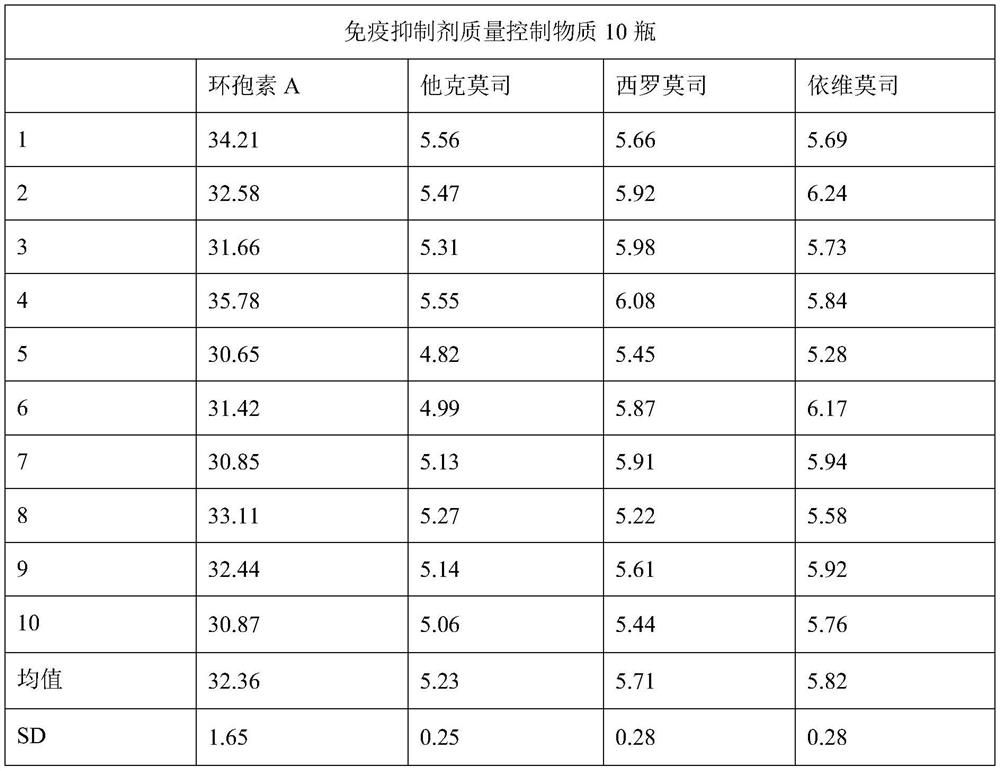
[0047] Collect whole blood without jaundice, chyle, and hemolysis, select the whole blood samples with negative infectious disease screening results (HIV, hepatitis B, syphilis, hepatitis C) for blood type testing, and select 100mL of type A blood to mix evenly for quality control Product preparation; add 5g of PVP40, stir until completely dissolved; add 0.5g of tert-butanol, stir until completely dissolved.
[0048] After testing, the whole blood does not contain cyclosporine A, tacrolimus, sirolimus, and everolimus, and the prepared quality control product contains cyclosporine A 35ng / mL, tacrolimus, sirolimus Limus and everolimus 6ng / mL, to which an appropriate amount of standard stock solution needs to be added.
[0049] According to the concentration of tacrolimus, sirolimus, and everolimus standard stock solution, the volume of whole blood, and the concentration requirements of quality control products, calculate the volume of the standard stock solution that needs to be...
example 2
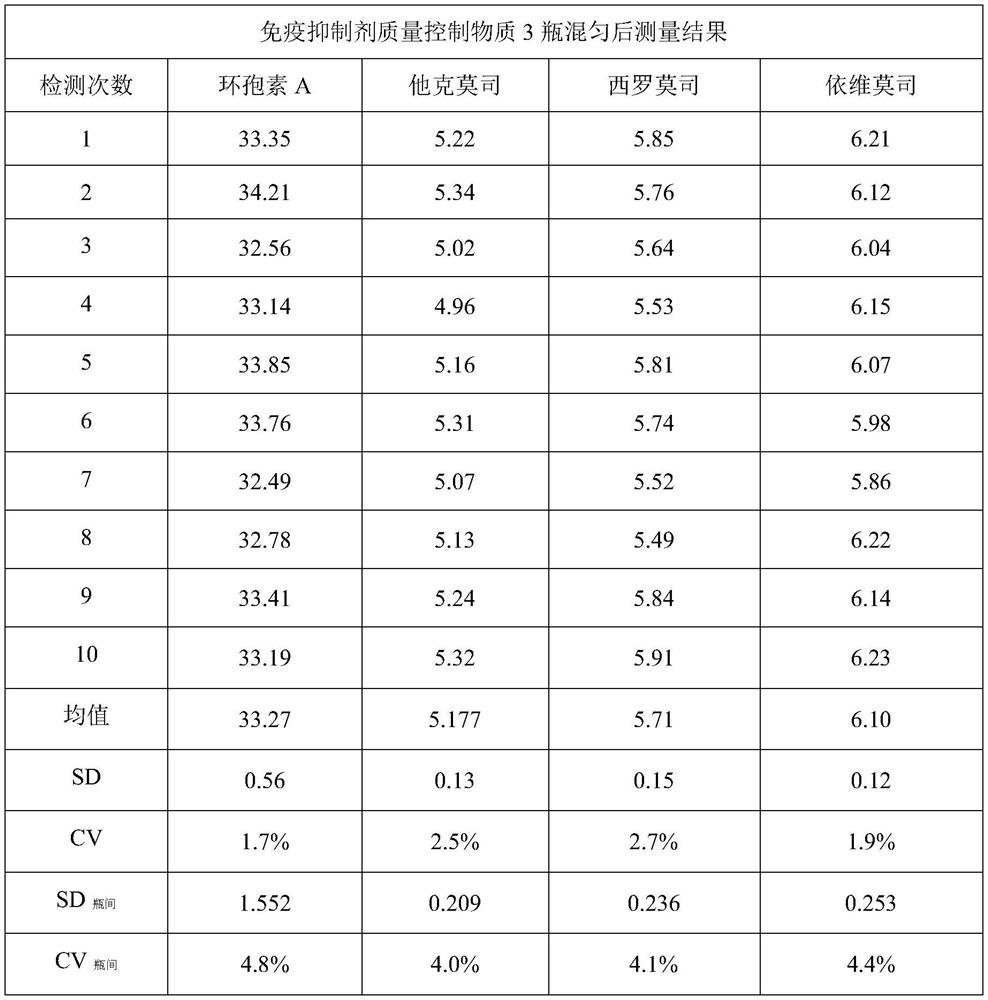
[0051] Collect whole blood without jaundice, chyle, and hemolysis, and select whole blood samples with negative infectious disease screening results (HIV, hepatitis B, syphilis, hepatitis C) to mix, add 20mg EDTA-K 2 Stir until completely dissolved, add 10g of PVP40, stir until completely dissolved; add 2.5g of tert-butanol, stir until completely dissolved.
[0052] After testing, the whole blood does not contain cyclosporine A, tacrolimus, sirolimus, and everolimus, and the prepared quality control product contains cyclosporine A 50ng / mL, tacrolimus, sirolimus Limus and everolimus 10ng / mL, to which an appropriate amount of standard stock solution needs to be added.
[0053] According to the concentration of tacrolimus, sirolimus, and everolimus standard stock solutions, the volume of whole blood, and the concentration requirements of quality control substances, calculate the volume of the standard stock solution that needs to be added, and pipette it to a clean In the beaker...
example 3
[0055] Collect whole blood without jaundice, chyle, and hemolysis, select the whole blood samples with negative infectious disease screening results (HIV, hepatitis B, syphilis, hepatitis C) and mix them, add 113mg of heparin and stir until completely dissolved, add 10g of PVP40, and stir until Dissolve completely; add 2.5g of tert-butanol and stir until completely dissolved.
[0056] After testing, the whole blood does not contain cyclosporine A, tacrolimus, sirolimus, and everolimus, and the quality control product contains cyclosporine A 100ng / mL, tacrolimus, sirolimus Limus and everolimus 20ng / mL, to which an appropriate amount of standard stock solution needs to be added.
[0057]According to the concentration of tacrolimus, sirolimus, and everolimus standard stock solution, the volume of whole blood, and the concentration requirements of quality control products, calculate the volume of the standard stock solution that needs to be added, and pipette it to a clean In the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com