Prednisone acetate tablets and preparation method thereof
A technology of prednisone acetate tablets and prednisone acetate, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of difficult absorption of tablets, and achieve the appearance Complete and smooth, convenient process steps, rapid dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
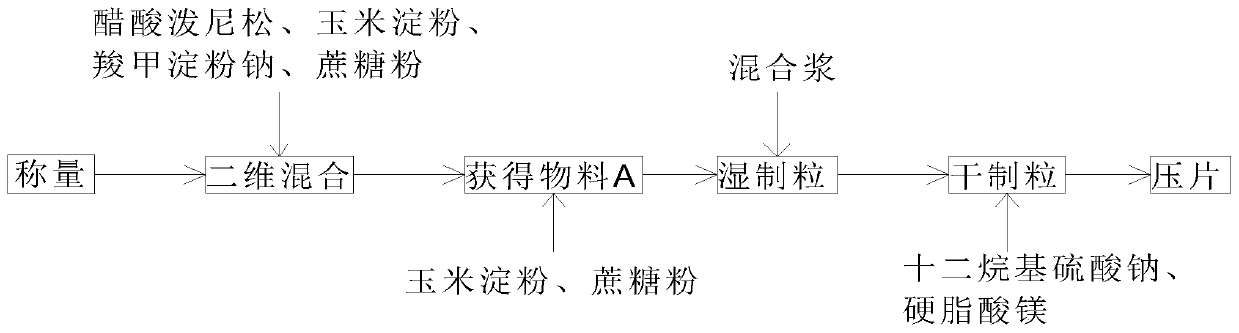
[0031] The embodiment of the present invention provides a kind of prednisone acetate tablet, comprises following composition by weight: prednisone acetate 30kg, cornstarch 459kg, sucrose 202.8kg, sodium starch glycolate 10kg, sodium lauryl sulfate 4.8kg, stearic acid Magnesium 5kg.
[0032] The quality parameter of prednisone acetate tablet is: moisture≤5.0%, content=107.0% (because there is certain error in the total mass of prednisone acetate tablet in the tablet process, cause the active ingredient in the prednisone acetate tablet to also exist Deviation, causing the content of some active ingredients to be greater than 100%), friability ≤ 0.8%, dissolution rate limited to 75% of the labeled amount, complete and smooth appearance, and uniform color.
[0033] The preparation method of prednisone acetate sheet, comprises the following steps:
[0034] S1. Weigh the raw materials of each component in the weighing workshop in parts by weight.
[0035] S2. Weigh 30kg of prednis...
Embodiment 2
[0042] The embodiment of the present invention provides a kind of prednisone acetate tablet, comprises following composition by weight percentage: prednisone acetate 45kg, cornstarch 688.5kg, sucrose 300kg, sodium starch glycolate 15kg, sodium lauryl sulfate 7.2kg, stearin Magnesium acid 7.5kg.
[0043] The quality parameters of the prednisone acetate tablet are: moisture≤5.0%, content=93%, friability≤0.8%, the dissolution rate is limited to 75% of the marked amount, the appearance is complete and smooth, and the color is uniform.
[0044] The preparation method of prednisone acetate sheet, comprises the following steps:
[0045] S1. Weigh the raw materials of each component in the weighing workshop in parts by weight.
[0046] S2. Weigh 45kg of prednisone acetate, 15kg of carboxymethyl starch sodium, and 300kg of cornstarch by weight, and place them in a two-dimensional mixer for mixing and stirring for 30min while controlling the environmental conditions: temperature 16-28°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com