Preparation method of prednisone
A technology of prednisone and hydrocortisone acetate, applied in the direction of microorganism-based methods, biochemical equipment and methods, steroids, etc., can solve the problems that are not suitable for industrial production, difficult to treat cyanide-containing wastewater, and potential safety hazards, etc. problems, to achieve the effect of being conducive to safe production, low operating cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
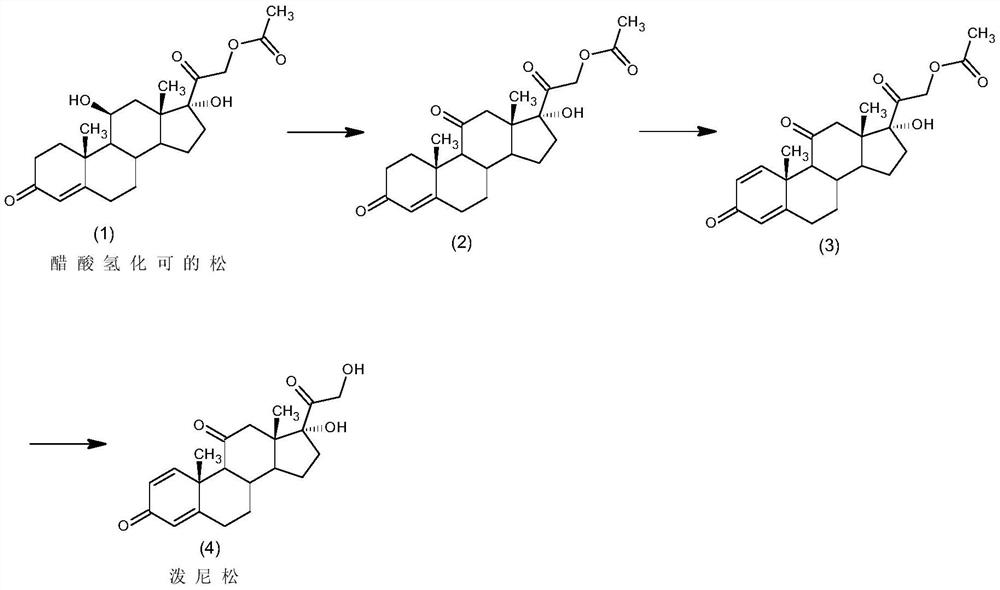
[0029] A kind of preparation method of embodiment 1 prednisone, comprises the steps:
[0030] 1) Oxidation reaction: join 20g hydrocortisone acetate (1) in 300ml dichloromethane, add 200ml50% acetic acid aqueous solution, 10g manganese sulfate, 4ml70% chromium trioxide aqueous solution, control temperature 0 ℃ and stir reaction, after reaction finishes , adding 20ml of 20% aqueous sodium bisulfite solution, washing with water until neutral, concentrating, water analysis, filtering, and drying to obtain 18.9g of intermediate 2;
[0031] 2) Dehydrogenation reaction: 18.9 g of intermediate (2) obtained in step 1) was passed through the traditional Arthrobacter Simplex By-2-13 fermentation method to obtain 17.0 g of intermediate 3;
[0032] 3) Hydrolysis reaction: Add the 17.0 intermediate (3) obtained in step 2) into a mixed solvent of 85ml of dichloromethane and 85ml of methanol, add 17ml of 10% triethylamine aqueous solution, and control the temperature at 30°C to stir the reac...
Embodiment 2
[0033] A kind of preparation method of embodiment 2 prednisone, comprises the steps:
[0034] 1) Oxidation reaction: 20g hydrocortisone acetate (1) joins in 150ml dichloroethane, adds 100ml60% acetic acid aqueous solution, 5g manganese sulfate, 20ml 30% chromium trioxide aqueous solution, controls temperature 15 ℃ and stirs reaction, and reaction finishes Afterwards, 10ml of 30% potassium sulfite aqueous solution was added, washed with water until neutral, concentrated, water separated, filtered, and dried to obtain 18.8g of intermediate 2;
[0035] 2) Dehydrogenation reaction: 18.8g of intermediate (2) obtained in step 1) was passed through the traditional Arthrobacter Simplex By-2-13 fermentation method to obtain 16.8g of intermediate 3;
[0036] 3) Hydrolysis reaction: the 16.8 intermediate (3) obtained in step 2) is added to a mixed solvent of 160ml of chloroform and 100ml of isopropanol, 80ml of 1% aqueous sodium hydroxide solution is added, and the temperature is control...
Embodiment 3
[0037] A kind of preparation method of embodiment 3 prednisone, comprises the steps:
[0038] 1) Oxidation reaction: 20g hydrocortisone acetate (1) joins in 60ml chloroform, adds 60ml90% acetic acid aqueous solution, 2g manganese chloride, 10ml50% chromium trioxide aqueous solution, controls temperature 30 ℃ and stirs reaction, after reaction finishes , adding 4ml of 50% sodium sulfite aqueous solution, washing with water until neutral, concentrating, water analysis, filtering and drying to obtain 19.2g of intermediate 2;
[0039] 2) Dehydrogenation reaction: 19.2 g of intermediate (2) obtained in step 1) was passed through the traditional Arthrobacter Simplex By-2-13 fermentation method to obtain 17.0 g of intermediate 3;
[0040] 3) Hydrolysis reaction: Add the 17.0 intermediate (3) obtained in step 2) into 680ml of ethanol, add 34ml of 5% potassium carbonate aqueous solution, and control the temperature at 15°C to stir the reaction. After the reaction, add 35% acetic acid a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com