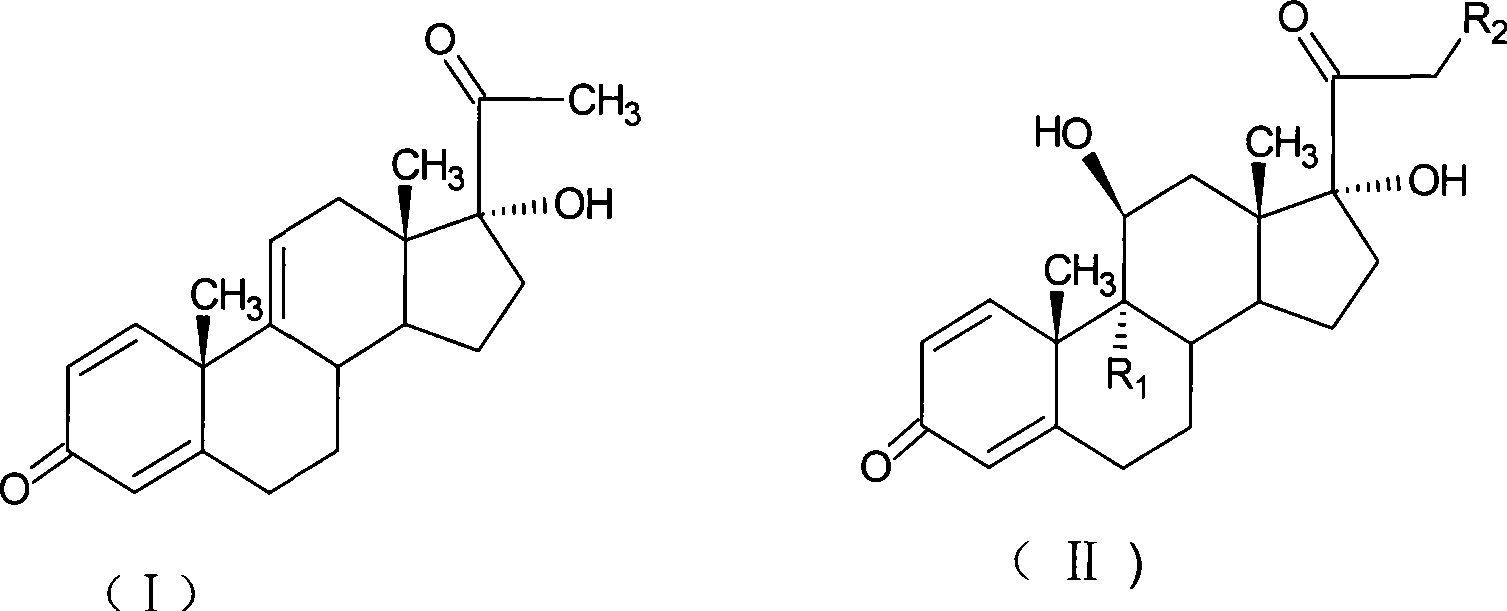
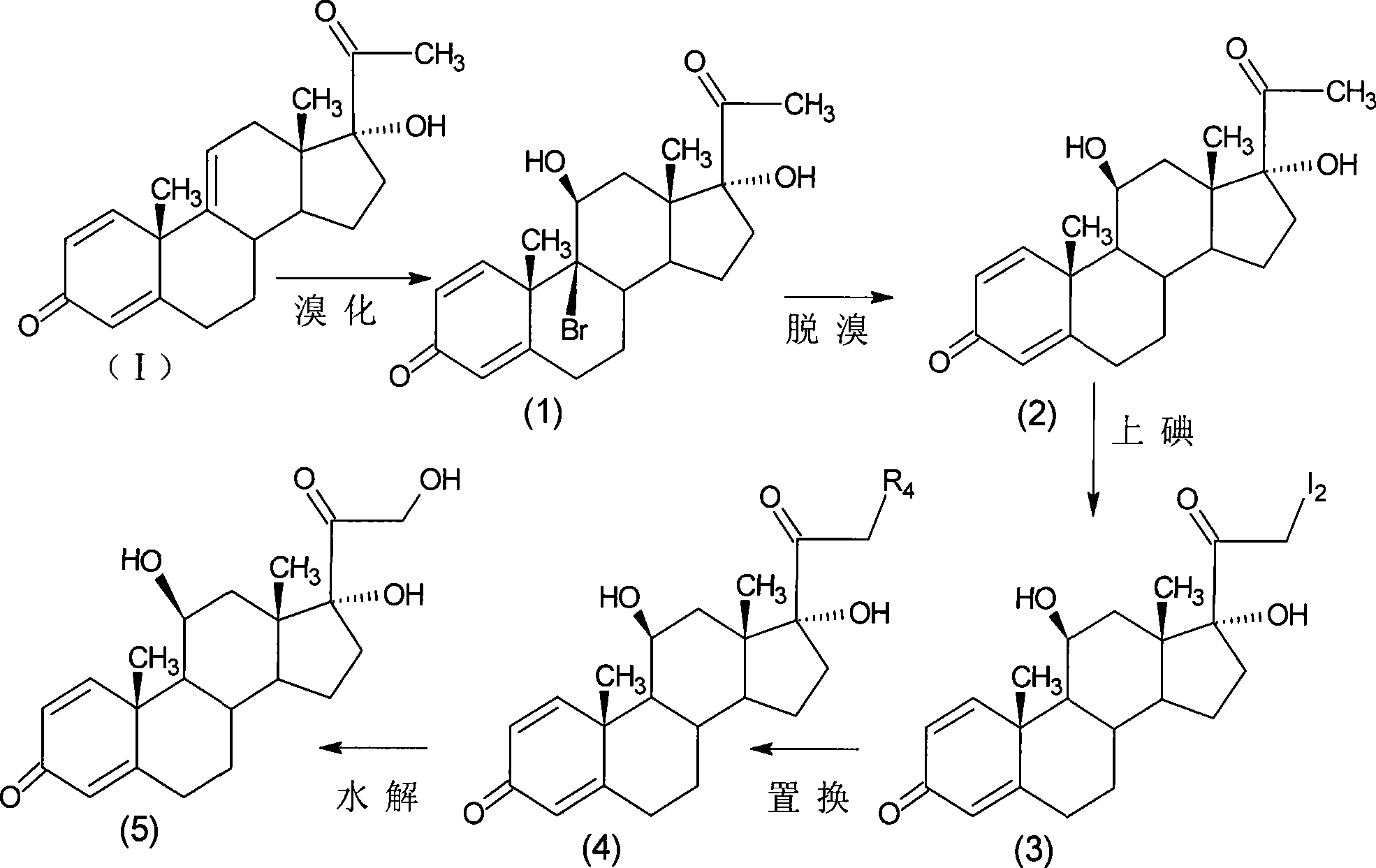
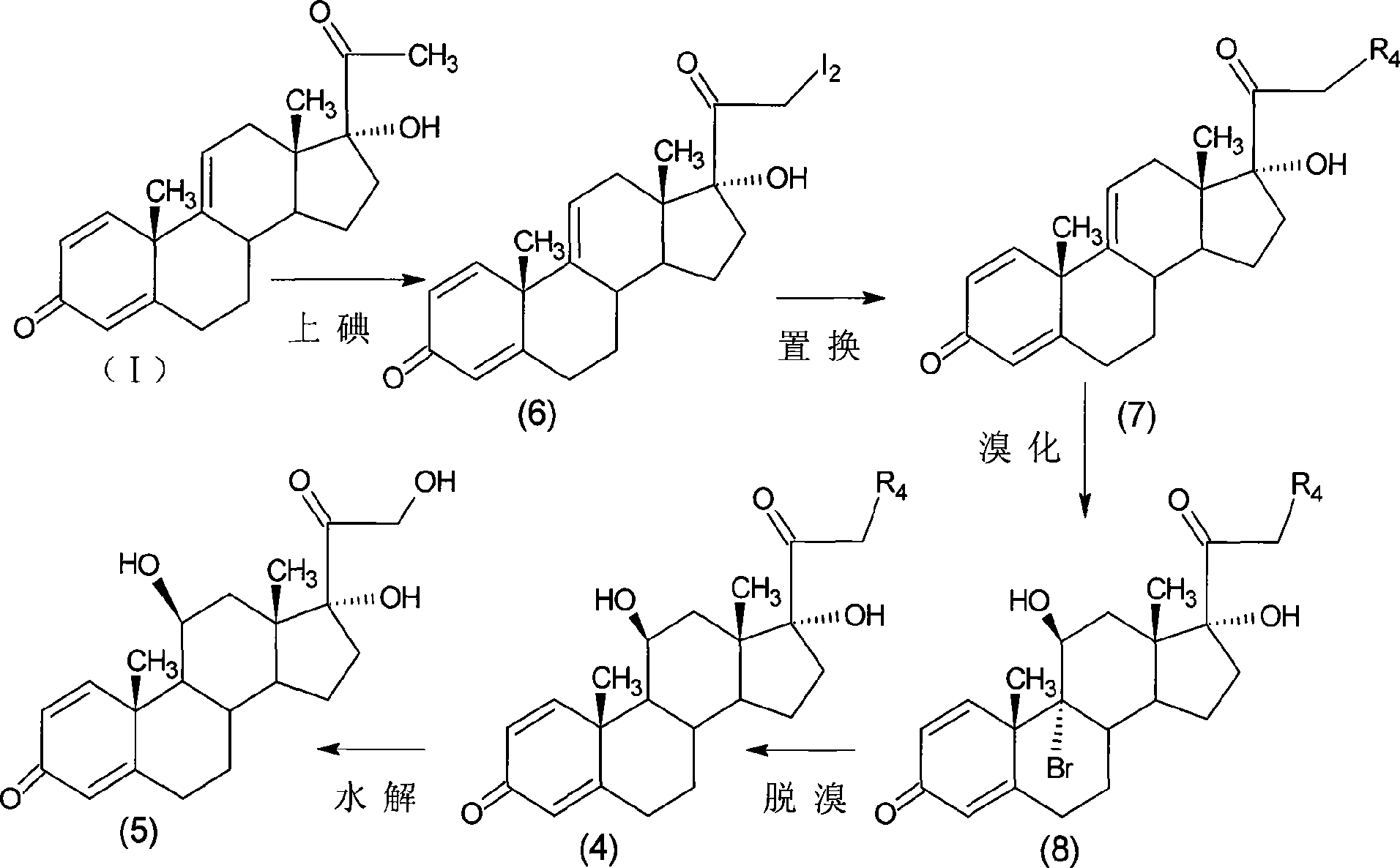
Preparation of metacortandralone and derivatives thereof
A compound and reactant technology, applied in the field of preparation of steroid compounds, can solve the problems of high cost and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of embodiment one prednisolone and prednisolone ester
[0082] Bromination reaction: 9α-bromo-11β, 17α-dihydroxy-1,4-diene-pregna-3,20-dione;
[0083] Add 10g of 17α-hydroxy-1,4,9-triene-pregna-3,20-dione (CN1896090), 100ml of acetone into the reaction flask, stir, cool down to 0°C, and add NBS within 30 minutes 9g, kept at 5-10°C for 2.5 hours, added 10% sodium carbonate aqueous solution to neutralize to PH=6.5, diluted in water, filtered and dried to obtain 12.1g of bromide (1).
[0084] Debromination reaction: 11β, 17α-dihydroxy-1,4-diene-pregna-3,20-dione;
[0085] Prepare the reducing agent: take 10g of chromium particles in the reaction bottle, pass nitrogen gas, add 10ml of concentrated hydrochloric acid, react at room temperature for 20 minutes, and set aside.
[0086] Add 12.1g of bromide (1) and 60ml of dimethylformamide into another reaction flask, blow nitrogen, cool down to 10°C, slowly add the prepared chromium reducing agent, react for 3...
Embodiment 2
[0093] The preparation of embodiment two prednisolone and prednisolone ester
[0094] Bromination reaction: 9α-bromo-11β, 17α-dihydroxy-1,4-diene-pregna-3,20-dione;
[0095] Add 10g of 17α-hydroxy-1,4,9-triene-pregna-3,20-dione (CN1896090) and 50ml of tetrahydrofuran into the reaction flask, stir, cool down to 0°C, and divide into two parts within 30 minutes. Bromocyanoacetamide 7g, kept at 5-10°C for 1.5 hours, added 10% sodium bicarbonate aqueous solution to neutralize to pH=6.5, diluted in water, filtered, dried to obtain 12.2g of bromide (1).
[0096] Debromination reaction: 11β, 17α-dihydroxy-1,4-diene-pregna-3,20-dione;
[0097] Add 12.2g of bromide (1) and DMF110ml into the reaction flask, pass nitrogen, heat and control the temperature at 80-90°C, quickly drop 20ml of tributyltin hydride as a reducing agent, react for 1 hour, cool down to 30°C, pour 700ml of saturated Dilute in sodium chloride solution, stir for 1 hour, let stand for 1 hour, filter, wash with water u...
Embodiment 3
[0104] The preparation of embodiment three prednisolone and prednisolone ester
[0105] Iodine reaction: 21-diiodo-17α-hydroxy-1,4,9-triene-pregna-3,20-dione;
[0106] Add 160ml of methanol and 6g of calcium oxide into the reaction flask, add 90ml of methanol solution and 8.2g of anhydrous calcium chloride into another volumetric flask, take out 1 / 4 after dissolving, add to the reaction flask, and dissolve the rest 15.0g of iodine particles, Add 10 g of 17α-hydroxy-1,4,9-triene-pregna-3,20-dione (CN1896090) into the reaction flask, fill with nitrogen, control the temperature at 0±5°C, add iodine solution dropwise, about 3 After 1 hour of dripping, after another 1 hour of reaction, the reaction solution was diluted in 600ml of 2% ammonium chloride aqueous solution, stirred for 1 hour, left to stand for 1 hour, filtered, washed with water until neutral, and obtained the wet product iodide (6) , This product is unstable, no drying is required, and the storage time should not be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com