Prednisolone dihydrate as well as preparation method and application thereof
A technology of dihydrate and prednisolone, which is applied in the field of medicine, can solve problems such as high dehydration temperature, yellowing and deterioration of products, and high energy consumption, and achieve the effects of increasing yield, shortening time, and overcoming high temperature of baking material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 prednisolone dihydrate
Embodiment 1-1
[0048] Add 100g of crude prednisolone into a mixed solvent of methanol (200mL), dichloromethane (200mL) and purified water (60mL), heat to 35°C to dissolve and clarify, filter with suction to remove insoluble impurities, and use a constant pressure dropping funnel Add purified water (1000mL) to the mixture at a rate of 300mL / min for crystallization, mechanically stir for 1h, then cool down to 10°C at a rate of 30°C / h, filter with suction, and dry at 35°C to constant weight to obtain 97.2g of solid , the yield was 97.2%.
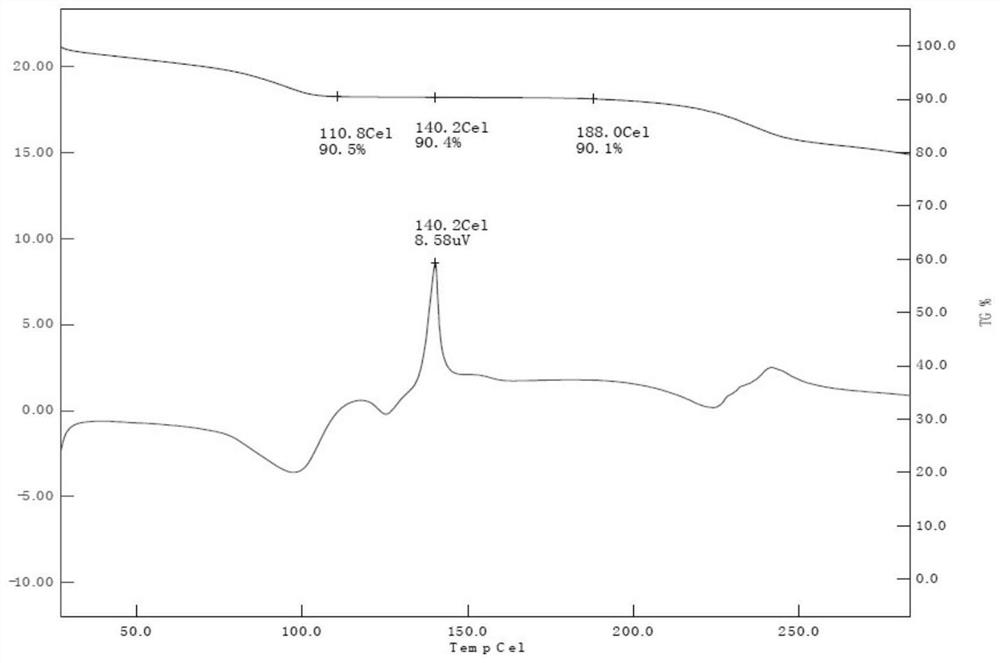
[0049] The obtained solid was analyzed by TG-DTA, and the weight loss was about 9.5%, which was confirmed to be prednisolone dihydrate. TG-DTA analysis such as figure 1 shown.
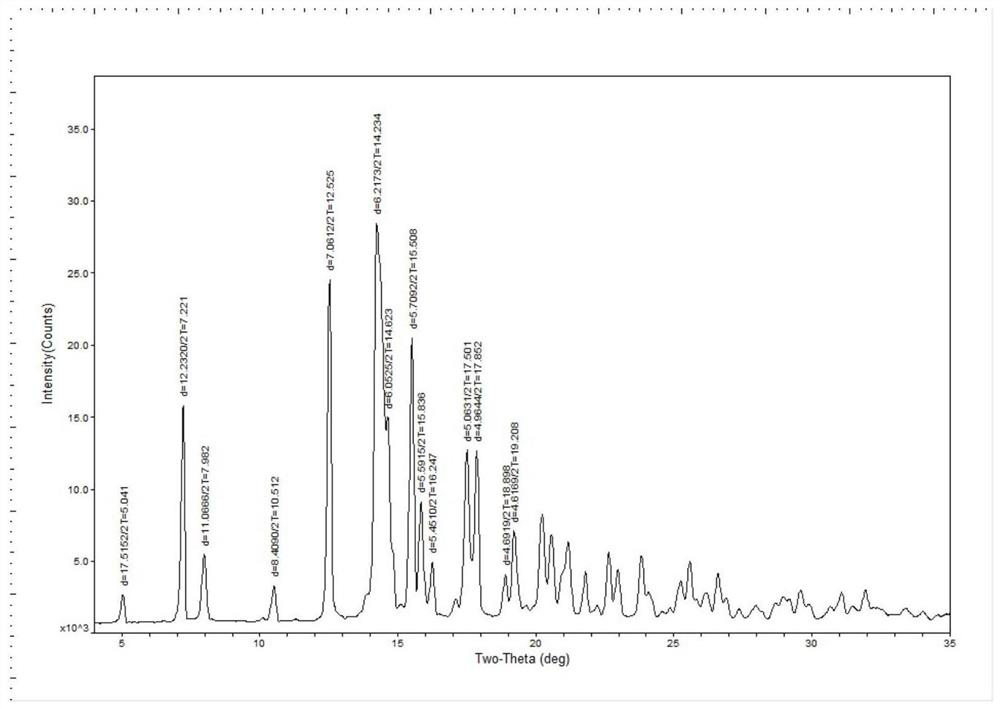
[0050] The obtained solid was subjected to X-ray diffraction measurement, and the measured characteristic peak positions were 2θ=7.2°±0.2°, 12.5°±0.2°, 14.2°±0.2°, 15.5°±0.2°, 16.2°±0.2°. X-ray diffraction measurements such as figure 2 shown.
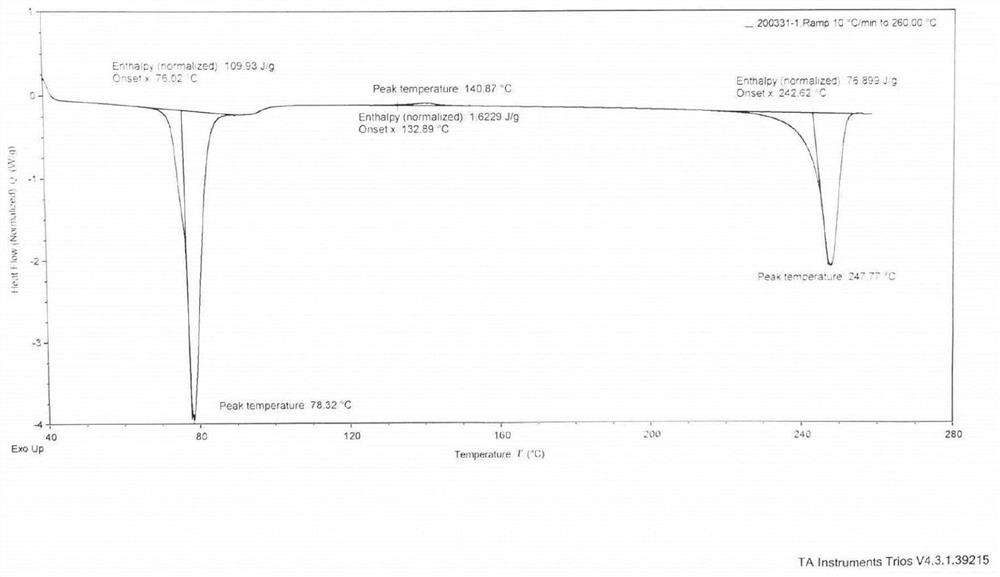
[0051] The obtained solid was subj...
Embodiment 1-2
[0053] Add 100g of crude prednisolone into a mixed solvent of ethanol (1000mL), ethyl acetate (200mL) and purified water (200mL), heat to 75°C to dissolve and clarify, filter with suction, remove insoluble impurities, and use a constant pressure dropping funnel Add purified water (100mL) to the mixture at a rate of 200mL / min for crystallization, mechanically stir for 2h, then cool down to 0°C at a rate of 20°C / h, filter with suction, and dry at 33°C to constant weight to obtain 97.5g of solid , the yield was 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com