Preparation method of prednisolone acetate
A technology of prednisolone acetate and sodium hypochlorite, which is applied in the fields of steroids and organic chemistry, can solve the problems of difficult separation in the post-treatment process, low concentration of substrate feeding, complicated production process, etc., and achieve good application prospects and unique system Odor reduction and high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
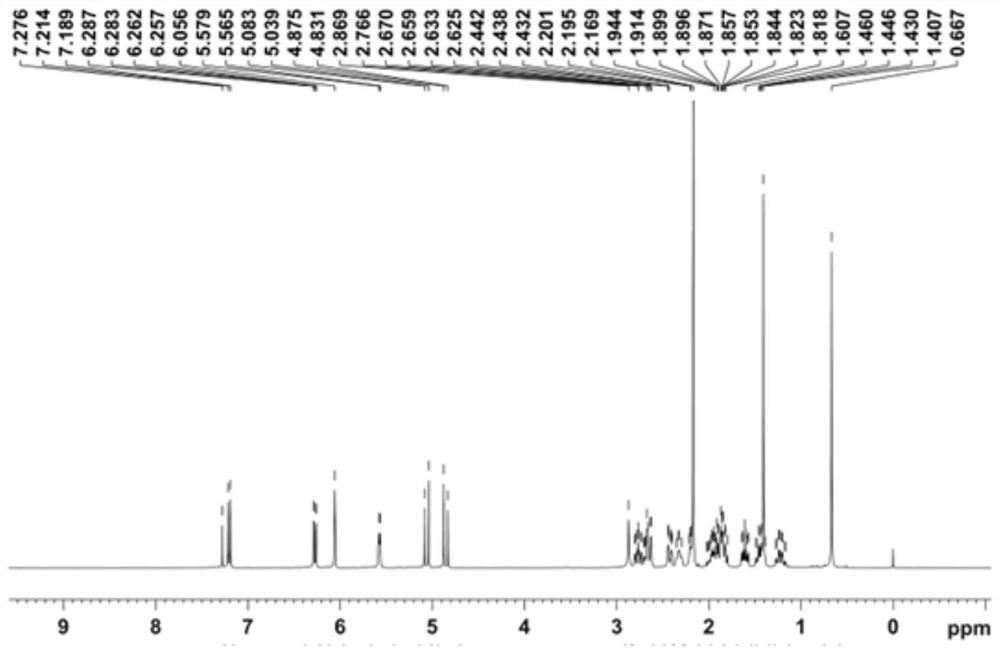
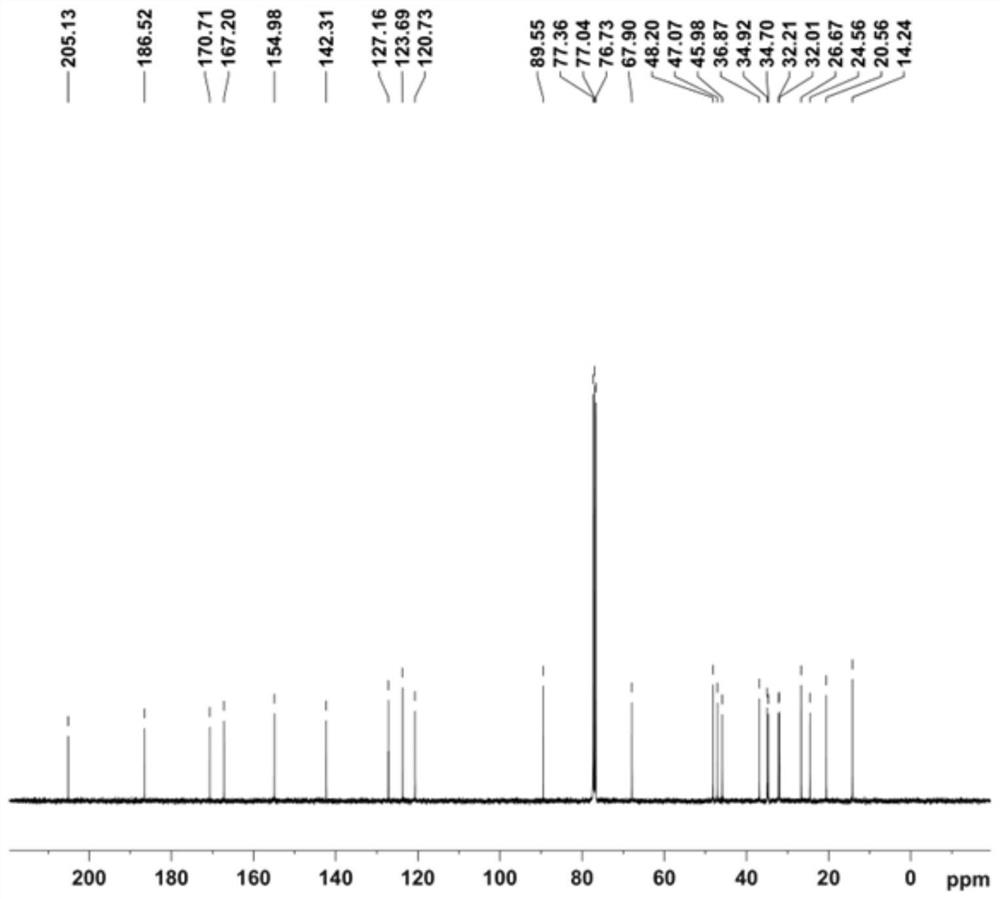
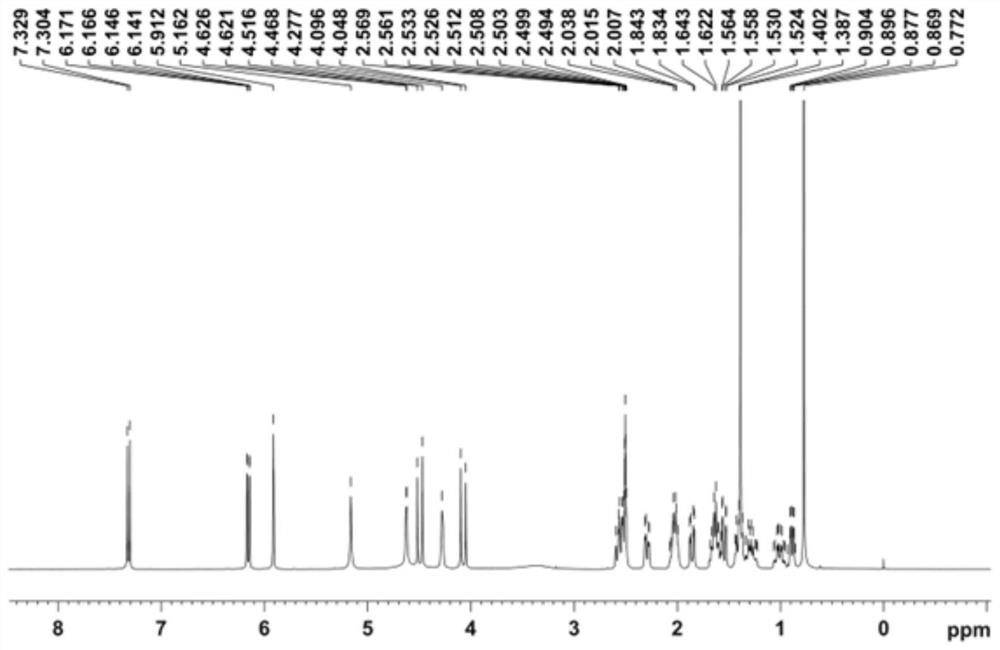
Image
Examples
preparation example Construction
[0034] One embodiment of the present invention provides a synthesis method of prednisolone acetate, comprising the following steps S10-S20.
[0035] Step S10: carry out bromination reaction on the compound of formula (I) to obtain the compound of formula (II); wherein, the structures of the compound of formula (I) and the compound of formula (II) are as follows:
[0036]
[0037] Step S20: subjecting the compound of formula (II) to a debromination reaction, and after post-treatment to obtain the compound of formula (III) prednisolone acetate; wherein, the post-treatment includes adding a sodium salt selected from sodium thiosulfate, sodium sulfite and sodium hypochlorite At least one, the structure of the compound of formula (III) is as follows:
[0038]
[0039] Sodium salt is added in the post-treatment to destroy solvents such as thioglycolic acid to achieve better impurity removal effect, and the special odor of the system is greatly reduced, not pungent, the process...
Embodiment 1
[0142] 1) Preparation of formula (IV) compound
[0143] 1.1 Incline cultivation
[0144] Nocardia was used as the strain, the medium composition and mass fraction were 1.3% glucose, 1.3% yeast extract and 2% agar, pH=7.0, cultured at 30°C for 3 days, and stored at 3-5°C for later use.
[0145] 1.2 Primary seed culture
[0146] The medium components are 0.3% beef extract and 0.5% peptone, pH=6.8, half of the slant medium is put into 100 ml primary seed medium, the shaker speed is controlled at 180rpm, and cultured at 30°C for 24h.
[0147] 1.3 Secondary seed culture
[0148] The composition of the culture medium is 1% glucose, 1% corn steep liquor, 1% peptone and 0.01% potassium dihydrogen phosphate, pH=7.0, the shaker speed is controlled at 180 rpm, and cultured at 30° C. for 16 hours.
[0149] 1.4 Feeding
[0150] Grind anecorta acetate (compound of formula V) to 200 mesh, weigh 120g, add water 360mL, Tween 80 3g, methanol 60mL, mix well and then feed into the secondary s...
Embodiment 2
[0176] Embodiment 2 is substantially the same as embodiment 1, and difference is that sodium thiosulfate is changed into sodium hypochlorite in the step 4) of embodiment 2, and other steps are identical with embodiment 1 with process parameter.
[0177] After calculation, the total yield of prednisolone acetate obtained in step 3) and step 4) is 91%, and the purity is 99.3%.
[0178] Prednisolone acetate yield=the molar weight of prednisolone acetate / the molar weight of formula (I) compound×100%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com