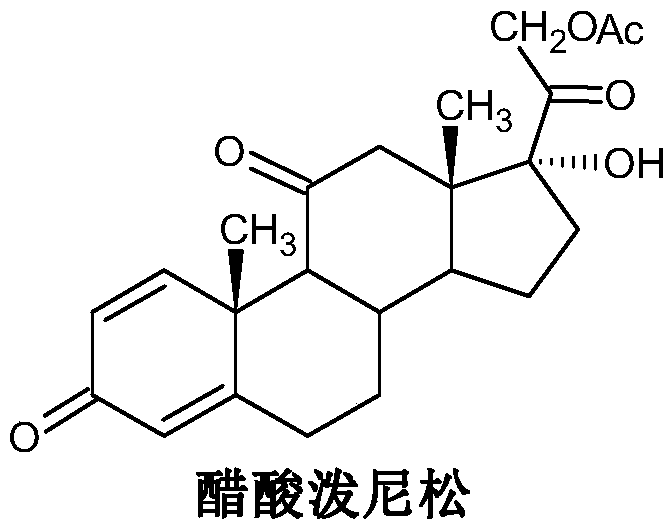
Preparation method of high-quality prednisone acetate and intermediate thereof
A technology of prednisone acetate and intermediates, which is applied in the field of preparation of high-quality prednisone acetate and intermediates thereof, can solve problems such as unsatisfactory conditions, and achieve the effects of simple and convenient operation, convenient operation and high feeding concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
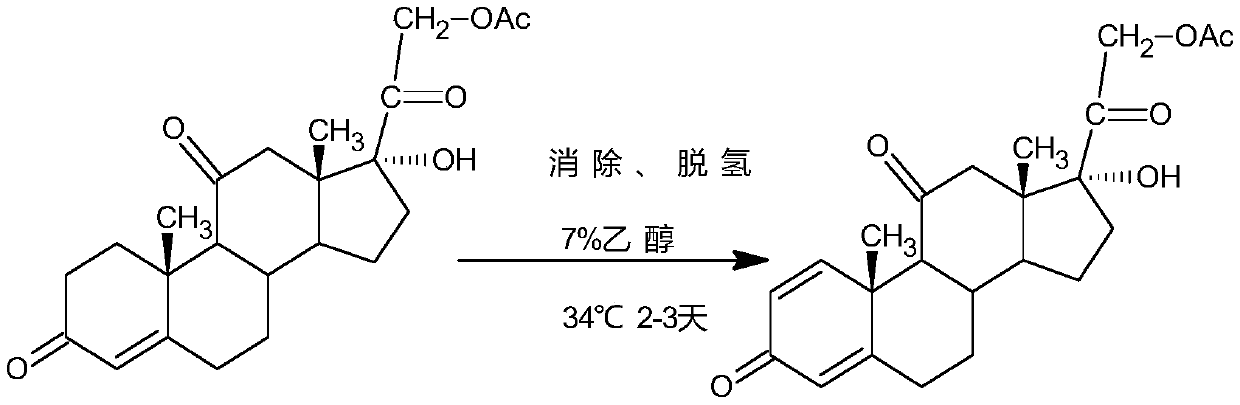
[0031] The invention provides a method for preparing high-quality prednisone acetate. The specific steps are as follows: taking epihydrocortisone as a raw material, first performing microbial dehydrogenation to obtain 11α, 17α, 21-trihydroxy-pregna-1,4 -diene-3,20-dione intermediate, and then through esterification to obtain 11α, 17α, 21-trihydroxy-pregna-1,4-diene-3,20-dione-21-acetate, and finally through Oxidation reaction makes prednisone acetate, and concrete technical route is as follows:
[0032] 1) Using epihydrocortisone as a raw material, dehydrogenating epihydrocortisone by simple Arthrobacter dehydrogenation, and preparing epihydrocortisone dehydrogenate as 11α, 17α, 21-trihydroxy-pregna-1,4-diene-3, 20-diketone, its concrete preparation steps are:
[0033] A. Culture of Arthrobacter simplex
[0034] Add culture medium into the shake flask, insert Arthrobacter simplex, culture at 28-35°C, 120-200rpm for 20-28 hours for later use;
[0035] B. Putting in raw mater...
Embodiment 1
[0048] A kind of preparation method of high-quality prednisone acetate intermediate, described prednisone acetate intermediate is 11α, 17α, 21-trihydroxy-pregna-1,4-diene-3,20-dione, Its concrete preparation steps are:
[0049] Add culture medium in shake flask, insert Arthrobacter simplex, 30 ℃, 160rpm culture 20-28 hour; Add raw material table hydrocortisone in cultured bacterial culture medium, add 7% ethanol, feeding amount 40g / L, 33°C, 180rpm after conversion for 1-2 days, TLC analysis and detection, until the conversion of raw materials is complete; separation and purification, the target product 11α, 17α, 21-trihydroxy-pregna-1,4-diene-3,20- Diketone has a purity of 99.16% as detected by HPLC.
Embodiment 2
[0051] A kind of preparation method of high-quality prednisone acetate and its intermediate, described prednisone acetate intermediate is 11α, 17α, 21-trihydroxy-pregna-1,4-diene-3,20-di Ketone, its specific preparation steps are:
[0052] 1) Culture of Arthrobacter simplex
[0053] Add culture medium in shake flask, insert Arthrobacter simplex, 30 ℃, 180rpm culture 20-28 hour; Add raw material table hydrocortisone in cultured bacterium culture medium, add 7% ethanol, feeding amount 40g / L, 33°C, 180rpm after conversion for 1-2 days, TLC analysis and detection, until the conversion of raw materials is complete; separation and purification, the target product 11α, 17α, 21-trihydroxy-pregna-1,4-diene-3,20- The diketone has a purity of 98.8% as detected by HPLC.
[0054] 2) Esterification reaction
[0055] Using the above-mentioned epihydrodehydrogenate as raw material, carry out esterification reaction to prepare intermediate 11α, 17α, 21-trihydroxy-pregna-1,4diene-3,20dione-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com