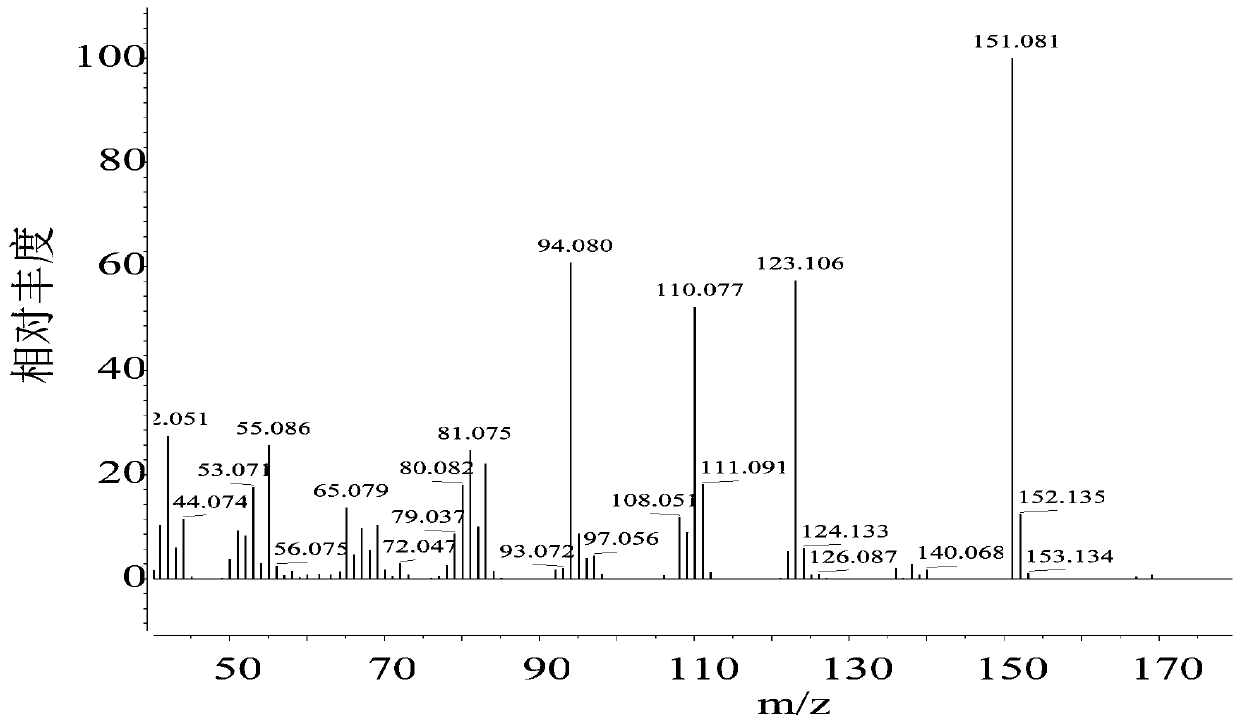
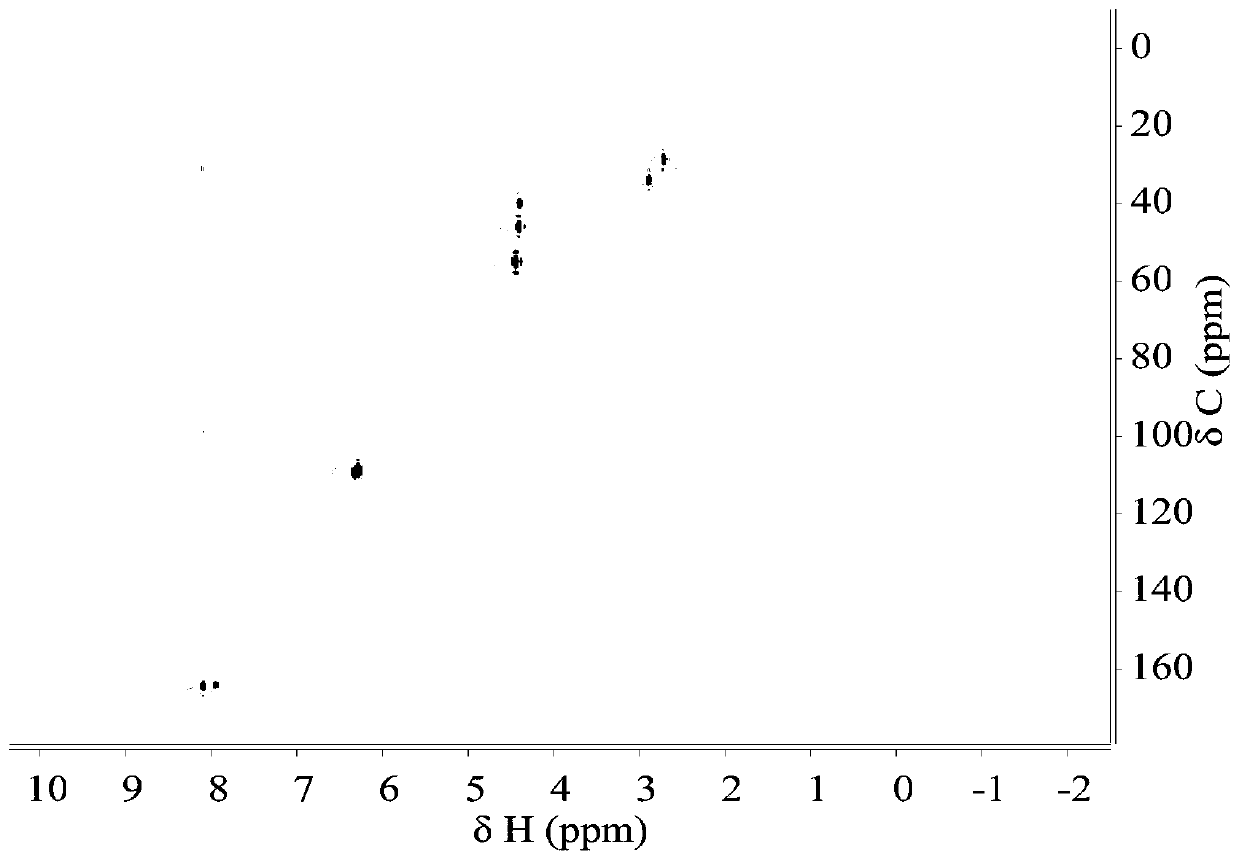
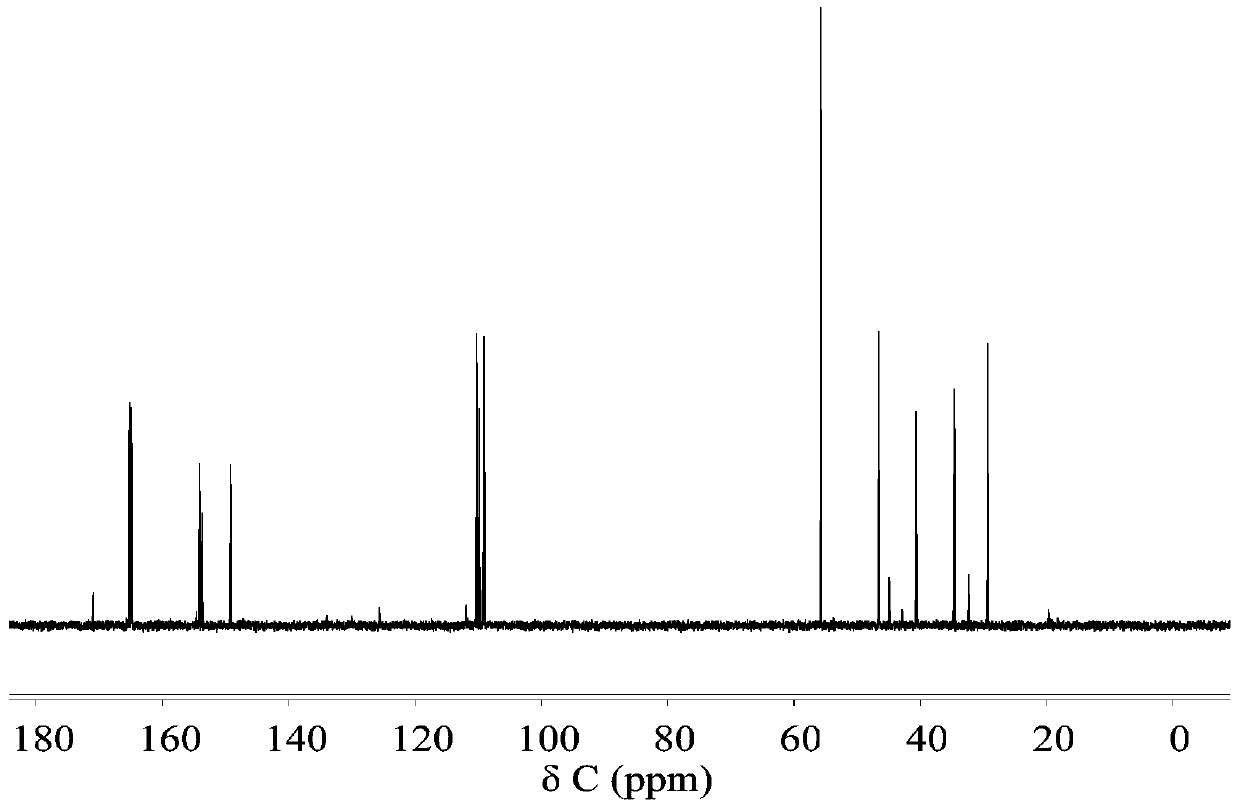
Synthesis of 2-n-methylaminomethyl-5-n-methyliminomethylfuran
A technology of methyliminomethylfuran and methylaminomethyl, applied in the field of organic compound synthesis, can solve problems such as few reports on furan synthesis, and achieve the effects of low equipment requirements, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 1.89g of HMF and 4.65g of methylamine into the 50mL reactor, shake and mix properly. Then add 25mL formic acid solution (7mol / L). Heat the reaction, the temperature is 170°C, the reaction time is 3h, and the mixture is mixed evenly by rotation at a speed of 300r / min. After the reaction is complete, put it in water to cool. The reaction solution was taken out, and the solvent was removed by rotary evaporation at 80°C until the solution mass no longer decreased. After the rotary evaporation, concentrated NaOH solution was added to the solution to adjust the pH to pH=12, and the residue was extracted several times with an equal volume of ethyl acetate, the extracts were combined, and the extractant was recovered by distillation under reduced pressure. Distill at 200°C under a pressure of 2000 Pa to obtain 2-N-methylaminomethyl-5-N-methyliminomethylfuran. As determined by GC, its purity is above 99%, and the product molar yield is 31.2%.
Embodiment 2
[0027] Add 1.89g of HMF and 9.03g of N-methylformamide solution (98%) into a 50mL reactor, shake and mix well. Then add 25mL formic acid solution (7mol / L). Heat the reaction, the temperature is 170°C, the reaction time is 3h, and the mixture is mixed evenly by rotation at a speed of 300r / min. After the reaction is complete, put it in water to cool. The reaction solution was taken out, and the solvent was removed by rotary evaporation at 80°C until the solution mass no longer decreased. After the rotary evaporation, concentrated NaOH solution was added to the solution to adjust the pH to pH=12, and the residue was extracted several times with an equal volume of ethyl acetate, the extracts were combined, and the extractant was recovered by distillation under reduced pressure. Distill at 200°C under a pressure of 2000 Pa to obtain 2-N-methylaminomethyl-5-N-methyliminomethylfuran. As determined by GC, its purity is above 99%, and the product molar yield is 35.0%.
Embodiment 3
[0029] Add 1.89g of HMF and 10.95g of N-methylacetamide solution (>99%) into a 50mL reactor, shake and mix properly. Then add 25mL formic acid solution (7mol / L). Heat the reaction, the temperature is 170°C, the reaction time is 3h, and the mixture is mixed evenly by rotation at a speed of 300r / min. After the reaction is complete, put it in water to cool. The reaction solution was taken out, and the solvent was removed by rotary evaporation at 80°C until the solution mass no longer decreased. After the rotary evaporation, concentrated NaOH solution was added to the solution to adjust the pH to pH=12, and the residue was extracted several times with an equal volume of ethyl acetate, the extracts were combined, and the extractant was recovered by distillation under reduced pressure. Distill at 200°C under a pressure of 2000 Pa to obtain 2-N-methylaminomethyl-5-N-methyliminomethylfuran. As determined by GC, its purity is above 99%, and the product molar yield is 32.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com