Method for preparing 16 alpha-hydroxyprednisolone
A technology of hydroxyprednisolone and hydroxyl, which is applied in the field of preparing 16α-hydroxyprednisolone, can solve the problems of being unsuitable for industrial production, high price of prednisolone, multiple side reaction impurities, etc., and is suitable for industrial production , good market prospects, high reactivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 116
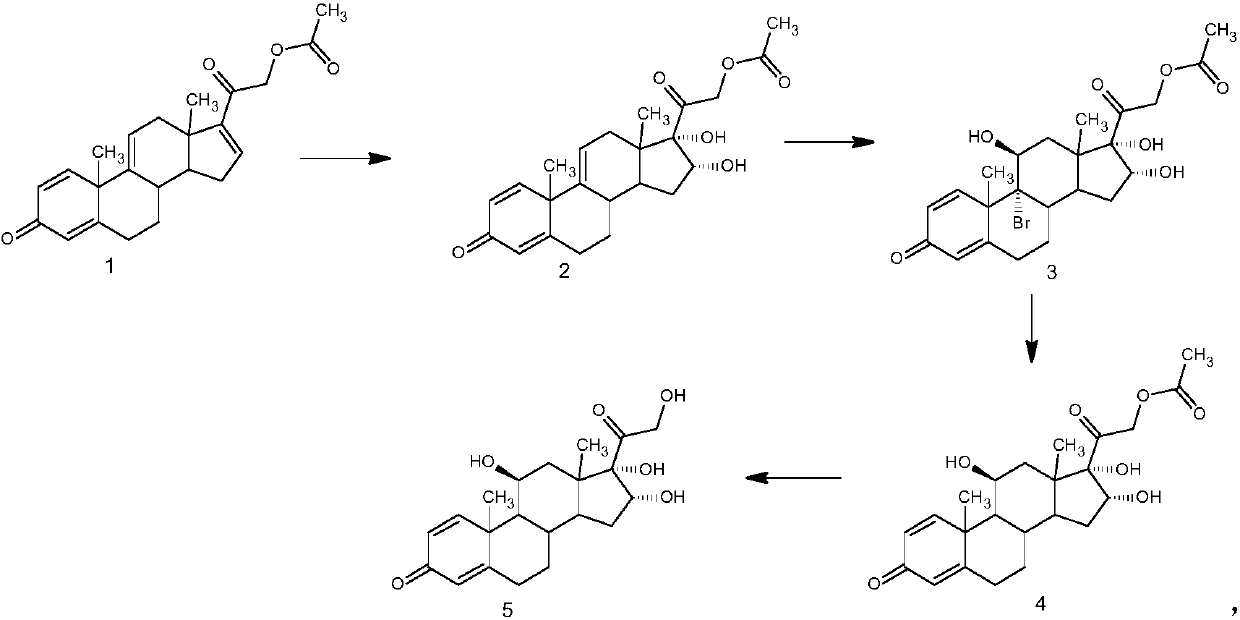
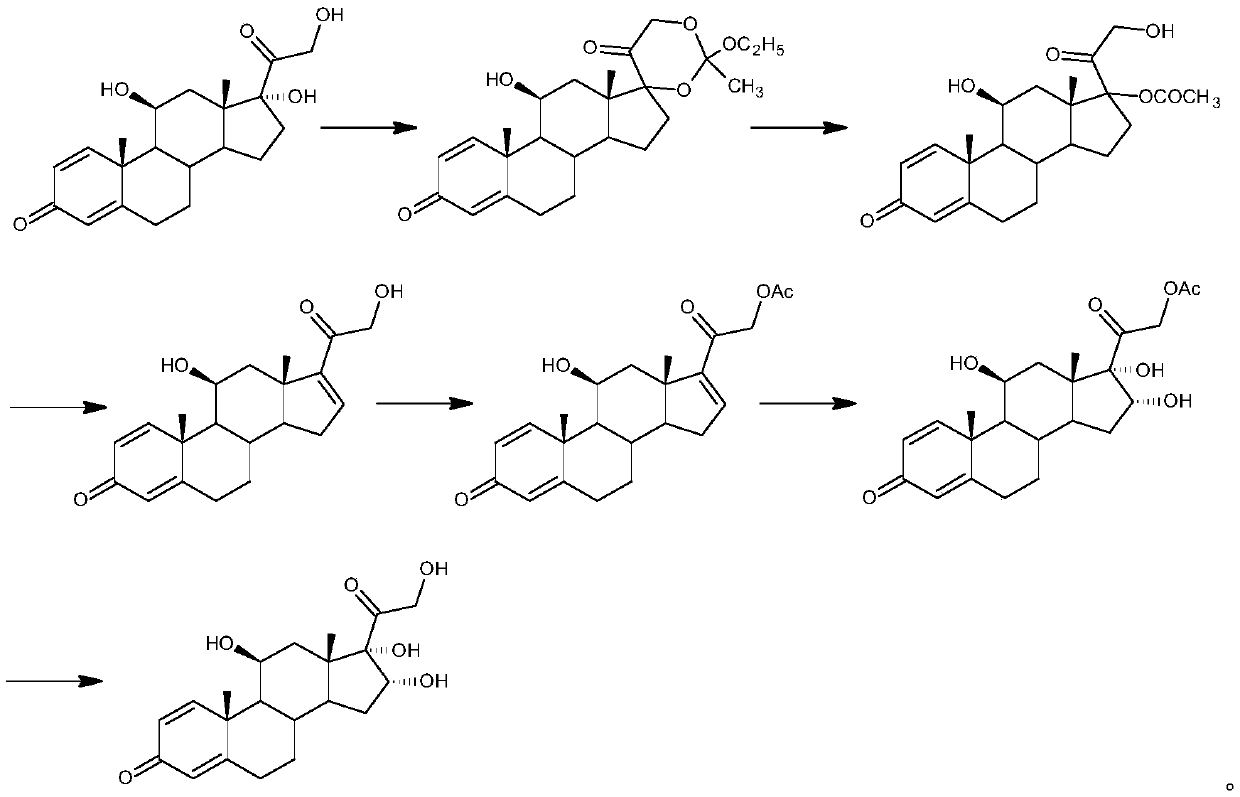
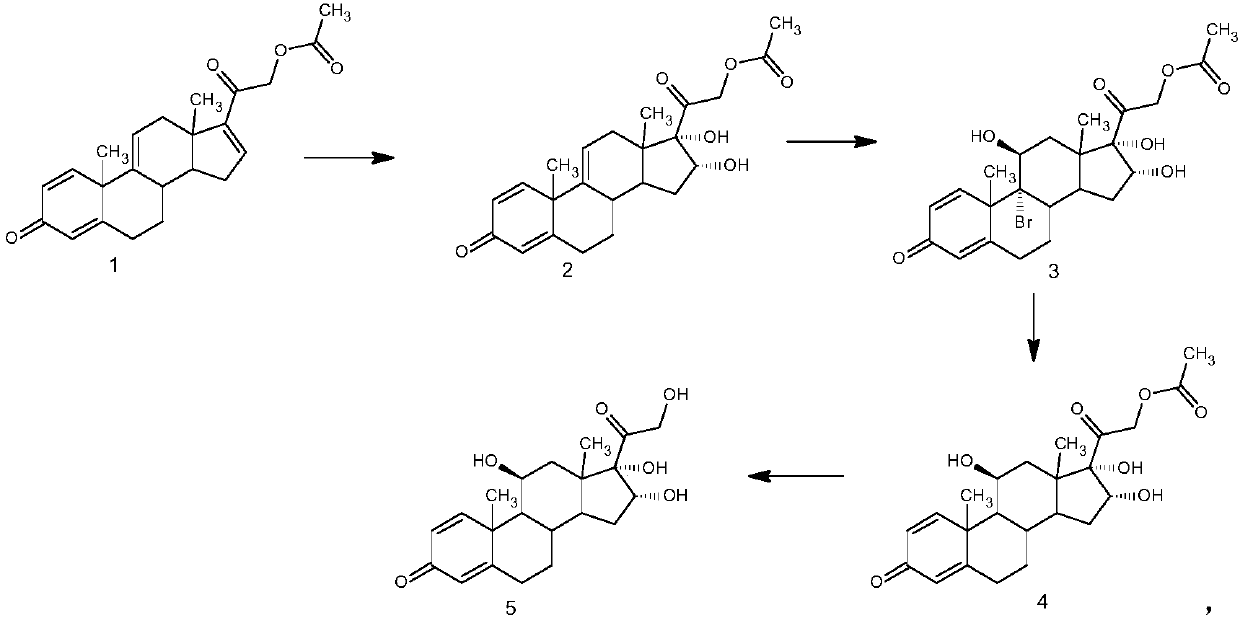
[0030] The preparation of example 1 16α-hydroxyprednisolone
[0031] 1. Oxidation reaction: 50 grams of 21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione-21-acetate (1) was dissolved in 2000 milliliters of acetone, and 10 Milliliter of formic acid, stirred and cooled to -10°C, added 300 ml of 8% potassium permanganate aqueous solution, stirred and reacted, after the reaction was completed, added 200 ml of 10% sodium bisulfite aqueous solution, filtered to collect the filtrate, concentrated to remove acetone, water analysis, Filter and dry to obtain 50 g of intermediate (2).
[0032] 2. Bromohydrin reaction: dissolve 50 grams of intermediate (2) in 2000 milliliters of butanone, add 120 milliliters of fluoroboric acid with a mass concentration of 2%, stir and cool down to 5° C., add 50 grams of dibromohydantoin and stir to react. After completion, 70 ml of 15% sodium sulfite aqueous solution was added to concentrate the butanone, water analysis, filtration, and drying to obtain...
example 216
[0035] The preparation of example 2 16α-hydroxyprednisolone
[0036] 1. Oxidation reaction: 50 grams of 21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione-21-acetate (1) was dissolved in 1500 milliliters of butanone, added 20 milliliters of oxalic acid, stirring and cooling to -5°C, adding 350 milliliters of 6% potassium permanganate aqueous solution, stirring and reacting, after the reaction was completed, adding 400 milliliters of 5% sodium bisulfite aqueous solution, collecting the filtrate by filtration, concentrating butanone, water Analysis, filtration, drying, to obtain 50.5 grams of intermediate (2).
[0037] 2. Bromohydrin reaction: dissolve 50.5 grams of intermediate (2) in 1500 milliliters of acetone, add 70 milliliters of perchloric acid with a mass concentration of 5%, stir and cool to 0°C, add 45 grams of N-bromosuccinimide and stir After the reaction was completed, 50 ml of 20% sodium sulfite aqueous solution was added, the acetone was concentrated, the water was ...
example 316
[0040] The preparation of example 3 16α-hydroxyprednisolone
[0041] 1. Oxidation reaction: 50 grams of 21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione-21-acetate (1) was dissolved in 1500 milliliters of dichloromethane, Add 15 ml of glacial acetic acid, stir and cool down to -20°C, add 320 ml of 7% potassium permanganate aqueous solution, stir for reaction, after the reaction is completed, add 100 ml of 20% sodium bisulfite aqueous solution, collect the filtrate by filtration, and concentrate to remove the organic solvent , water analysis, filtration, and drying to obtain 50.1 g of intermediate (2).
[0042] 2. Bromo-hydroxyl reaction: dissolve 50.1 grams of intermediate (2) in 2000 milliliters of acetone, add 50 milliliters of 10% methanesulfonic acid, stir and cool to -10°C, add 48 grams of N-bromosuccinimide Stir the reaction, after the reaction is completed, add 80 ml of 5% sodium sulfite aqueous solution, concentrate the organic solvent, water analysis, filtration, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com