Abiraterone acetate tablets and preparation method thereof
A technology of abiraterone acetate and acetic acid, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as cumbersome steps, easy doping with impurities, and poor consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0103] In order to further understand the content, features and effects of the present invention, the following examples are given, and detailed descriptions are given below with reference to the accompanying drawings.
[0104] The structure of the present invention will be described in detail below in conjunction with the accompanying drawings.
[0105]The composition and weight ratio of the abiraterone acetate tablet provided by the embodiment of the present invention consists of 30-50 abiraterone acetate, 0.4-2 hydroxypropyl cellulose, 1-4 sodium lauryl sulfate, and 40-40% microcrystalline cellulose. 60. Composition of crospovidone 1-8, magnesium stearate 0.5-2.5.
[0106] The working principle of the present invention is:
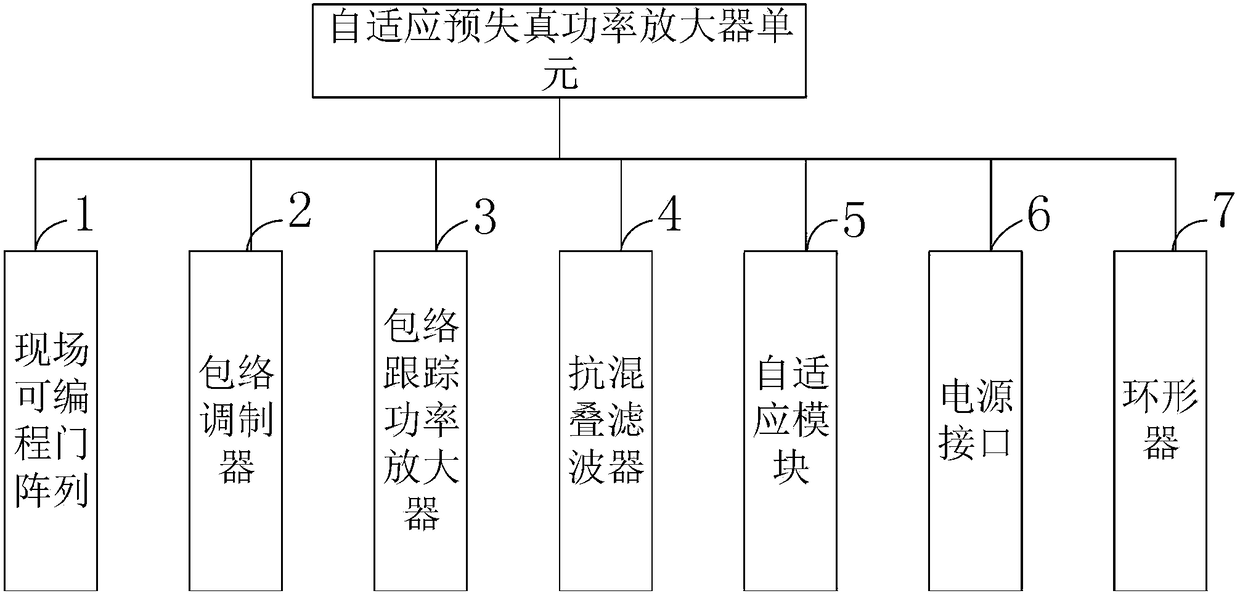
[0107] Such as figure 1 Shown, the preparation method of the Abiraterone acetate sheet that the embodiment of the present invention provides comprises the following steps:
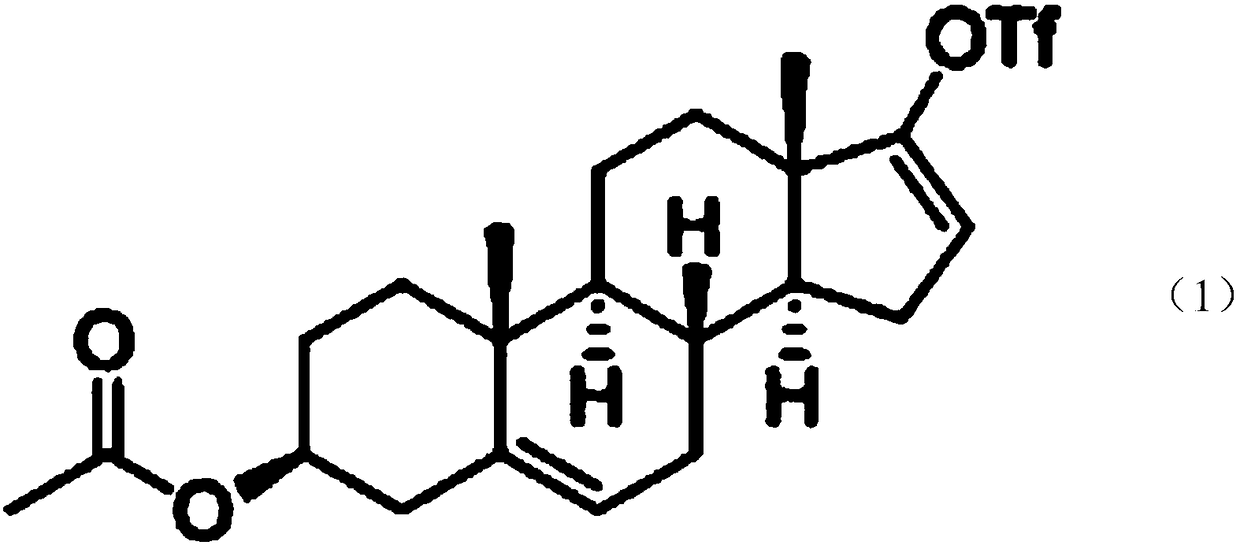
[0108] S101: Using dehydroepiandrosterone acetate as raw material, carry out...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com